7 Answers
Shreya Sharma
·2014-05-08 10:37:17
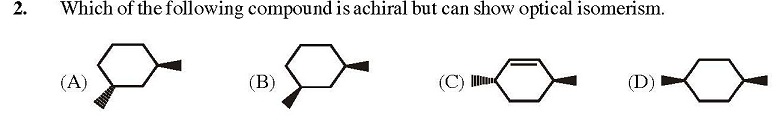
2. A
 Soumyadeep Basu Yeah, I also thought A would be the answer but it is given as B.Upvote·0· Reply ·2014-05-09 01:31:23
Soumyadeep Basu Yeah, I also thought A would be the answer but it is given as B.Upvote·0· Reply ·2014-05-09 01:31:23 Sourish Ghosh But A is chiral right? It has no point, plane, or line of symmetry.
Sourish Ghosh But A is chiral right? It has no point, plane, or line of symmetry. Soumyadeep Basu Yes it is. But can B show optical isomerism?
Soumyadeep Basu Yes it is. But can B show optical isomerism? Sourish Ghosh I think this might be an exception.
Sourish Ghosh I think this might be an exception.
Soumyadeep Basu
·2014-05-10 04:55:41
What about the other question?
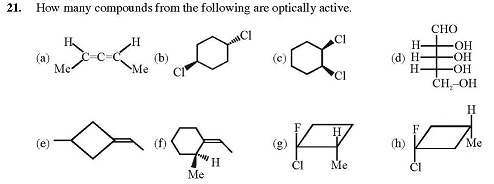
 Anik Chatterjee for the second one,is it 4..?
Anik Chatterjee for the second one,is it 4..?
 Soumyadeep Basu No, 5.
Soumyadeep Basu No, 5. Soumyadeep Basu Which ones are you getting?
Soumyadeep Basu Which ones are you getting?
Akshay Ginodia
·2014-05-12 04:14:57
A,B,D,F,G for the second one?
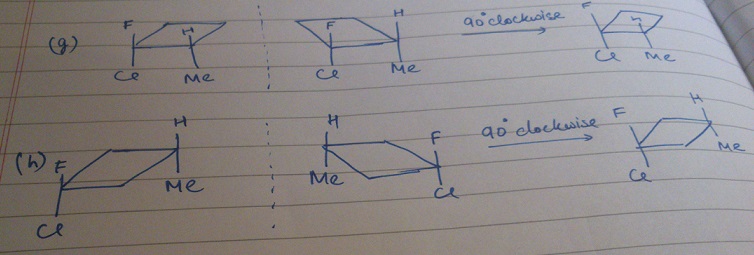
 Sourish Ghosh why not h?
Sourish Ghosh why not h? Niraj kumar Jha take the mirror image of 'h' and rotate it by 90(clockwise) we will get h.
Niraj kumar Jha take the mirror image of 'h' and rotate it by 90(clockwise) we will get h. Soumyadeep Basu Thanks.
Soumyadeep Basu Thanks. Aditya Agarwal What is wrong with E?
Considering option G, then if we rotate the mirror image by 90 clockwise, we get the initial molecule. So how is that active or where am I wrong?
Aditya Agarwal What is wrong with E?
Considering option G, then if we rotate the mirror image by 90 clockwise, we get the initial molecule. So how is that active or where am I wrong?
 Soumyadeep Basu Aditya, I don't think we can get G from its mirror image. If you are getting it, please can you upload an image? For E, I think you're right. Also, in the diagram, A is given to be planar, so how is it active?
Soumyadeep Basu Aditya, I don't think we can get G from its mirror image. If you are getting it, please can you upload an image? For E, I think you're right. Also, in the diagram, A is given to be planar, so how is it active? Anik Chatterjee isnt there a centre of symmetry in (b)?..at the centre if the ring?
Anik Chatterjee isnt there a centre of symmetry in (b)?..at the centre if the ring? Anik Chatterjee *of the ring
Anik Chatterjee *of the ring

Akshay Ginodia
·2014-05-15 06:29:58
Today i read that inspite of being achiral (e) is optically active bt i dnt knw d reason
 Soumyadeep Basu In E, consider the left methyl group to project out of the plane of the paper. Now if you rotate its mirror image to align all the other portions, you"ll see that the methyl group will project into the plane of the paper. I guess this might be the reason.
Soumyadeep Basu In E, consider the left methyl group to project out of the plane of the paper. Now if you rotate its mirror image to align all the other portions, you"ll see that the methyl group will project into the plane of the paper. I guess this might be the reason. Anik Chatterjee because the ring & the double bond aren't in the same plane...they are in 2 perpendicular planes...jus like 2 adjacent double bonds...like in allenes
Anik Chatterjee because the ring & the double bond aren't in the same plane...they are in 2 perpendicular planes...jus like 2 adjacent double bonds...like in allenes