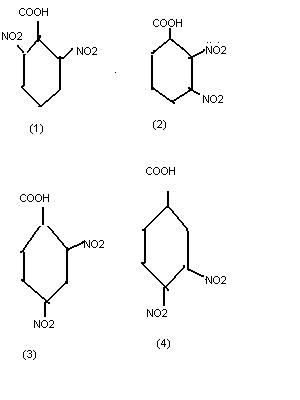
1
1you can proceed by checking the stability of the conjugate base...
 1
1yeah its the right answer...but how v cum to knw....???
 1
1because only electron withdrawing groups increase acidity of cooh group..
as we know NO2 is effective at ortho and para positions 2 and 4 have equal acidity..
as ortho group is nearer than para NO2 it has greter pulling efect compared to para position so 1 is given preference over 3
 39
39Ortho..para...bhai this is not a benzene ring..