 1
1Q1) SO3H ??? may be because it has highest preference among functional grps.
Q3) FeCl3 gives neutral soln. in water
Q5) Toluene reacts as a normal aromatic hydrocarbon towards electrophilic aromatic substitution.] The methyl group makes it around 25 times more reactive than benzene in such reactions. It undergoes smooth sulfonation to give p-toluenesulfonic acid, and chlorination by Cl2 in the presence of FeCl3 to give ortho and para isomers of chlorotoluene. It undergoes nitration to give ortho and para nitrotoluene isomers, but if heated it can give dinitrotoluene and
ultimately the explosive trinitrotoluene (TNT).
 106
1061. A (order is NR3+>NO2+ and then the rest)
2. D
3. A (strong base and strong acid give neutral soln)
4. A?
8. D
 24
24@manmay
ans1 rite,,rest wrong
explain 1st plz
@asish
ans1 wrong
rest all rite
but can u explain 4,8 ??
and in 2 will nitrobenzene be good starting compound though it is highly deactivating ??
 39
39It will be BF3 for Q4. The alkene's double bond acts as a nucleophile, donates electrons into boron's vacant orbitals(lewis acid action). This complex then acts as electrophile and attacks benzene, and when aromaticity is regained BF3 leaves.
In Q8, the para isomer of di-chlorobenzene has highest melting point because it fits best into its crystal lattice. Lattice energy is quite high and subsequently so is the melting point(as compound is more stable).
eure for Q2 have they given how many steps maximum or something? Because otherwise d) is the best option. We are only chlorinating once. If we try again it won't work as both chlorine and nitro groups are deactivating.
 1
1Q1) i wrote it on basis of priority of functional groups which is highest for sulphonic acid( -SO3H )group.
if my Q5) is rong then other ans. should be o- ,p- noitro toluene becoz may be dil. HNO3 i didn't see it was dilute.
 24
24@pritish
thx for Q8
btw cant it be SnCl4 for Q4 ??
and for Q2 ...nthing else given...
@manmay...
for Q1...i am not convinced by ur explaiantion ....how does priority define or depend on activating or deactivating power of functional gps.. ???
for Q5 u r still wrong....though i feel book is wrong....becoz ans 5 is given as benzoic acid
 29
29Ans1
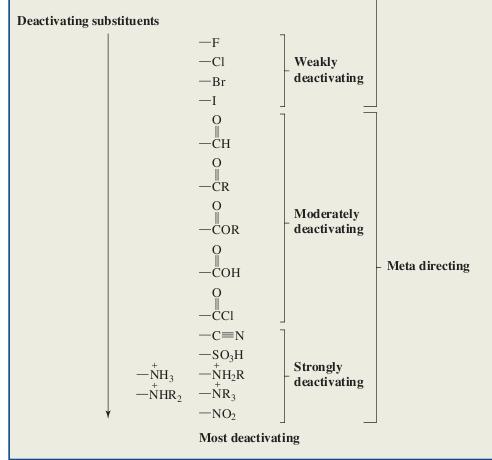
So according to this NO2 shud be the answer..
 39
39In Q7, bromoform will undergo alpha elimination by that base to give dibromocarbene. This will attack as electrophile on cyclopentadiene.
 23
23Q7 product will be bromobenzene

