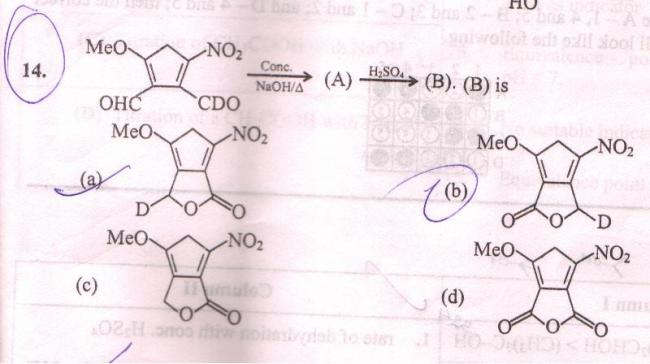
Ans A..see the carbon on the side of the NO2 grp shud be more reactive..so OH- will attack on that carbon..then there will be shift of D- to the other carbon (canninzaro reaction)...then H2SO4 will form the ester...
it is good that the BT ppl have given the options..otherwise i think OH- shud have extracted a proton from the carbon 1(the carbon which is the part of the ring and no grp is substituted on it) and the ring wud have become aromatic
14 Answers
Even I thought at first glance that the unsubstituted carbon will be deprotonated, govind...conc NaOH means hydroxide ion would act more readily as a base. Due to -I effects of NO2 and OCH3, that would be the best spot. None of the options reflect that however..
Instead it will be Cannizzaro oxidation reduction as you say. Then the two compounds will react in presence of acid to form ester.
the answer was given as (B) though..
govind's ans seems correct.. the one nearer to NO2 grp will be more reactive towards CR reaction.. (so it shud be A as i had marked)
yeah .. without seeing options ..I had also first thot that ring would become aromatic..
MULTI
Q2. Neopentyl bromide ---> OH- ----> (I)
Neopentyl bromide ---> H2O -----> (II)
(a) I(minor) is neopentyl alcohol formed by SN2
(b) II(major) is rearranged product by SN1
(c) I(major) is rearranged alkene by E1
(d) II(major) is rearranged alkene by E1
2.(a), (b), (c). Neopentyl substrates undergo SN1 more easily than SN2. The base is not bulky, but the substrate is, so the scales will tip in favour of elimination in (I).
@pritish.. if u see morrison boyd .... or sanyal...
they say that under SN2/E2 conditions, neopentyl halides react by very slow SN2 and no rearrangement takes place
while under SN1/E1 conditions .... both SN1/E1 occur but slow..
So ans shud be CD only naa?
Well..I'm going by my FIITJEE sir's notes, he's never been wrong in what he taught me, so I trust his judgement...
kk
but waht abt Q1..
It should be (a) naa?
another silly dbt
does phenol undergo fridel crafts reaction?
Of course, 1 is (a) and phenol does undergo friedel crafts. Aniline doesn't because of acid base reaction.
So Final answer of Qn. 1 is B....sry that in post 2 ..i sed the answer is A..but actually it shud be B... i missed that Rate Determining Step(R.D.S.) part...since H- shift is more favourable than D- shift..so attack of nucleophile is on the left carbon( on the side of -OCH3 grp)...in the question they shud have asked which is kinetically more favoured product ..but since the rate of reaction depends whole upon the rate of R.D.S. ..so product B is kinetically more favourable..... [1]
@govind:
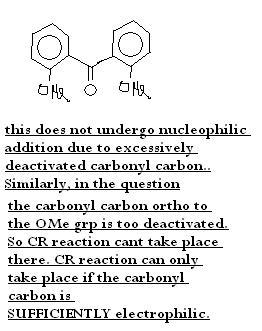
anyway got this confirmed from BT ppl.. also...
ans shud be (A)
@pritish: TCP and KCP can be applied everywhere provided the kinetic product and Thermo product are diff...
If same then no worries
also govind... notice that
Δ has been provided...
so it should be the thermodynamic product shudnt it?
@Asish...
i was wondering why those ppl gave the ans B..so i just saw the mechanism of Caninzzaro reaction to see if i am going wrong somewhere..there i found that Hydride transfer is the slow step so reaction rate shud depend on the R.D.S....So let's see wat experts hav to say in this question...
Well acc.. to my experience BT ppl commit only few mistakes..while the case of FIITJEE is totally different..