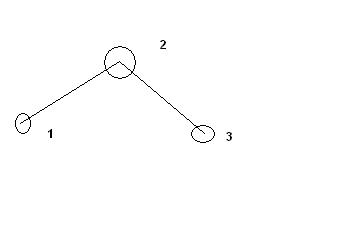
Here each FREE END represents a CH3....and the bent prt 2 shows CH2...the line joining 1 to 2 and 2 to 3 shows C-C bond ...
plz explain me how 2 give the bond line representation of compounds.Explain it with the help of examples. Also tell me the meaning of solid & dashed wedge formula.

Here each FREE END represents a CH3....and the bent prt 2 shows CH2...the line joining 1 to 2 and 2 to 3 shows C-C bond ...
and if two adjacent lines are given , it is a double bond and if three are there , it is a triple bond..and USUALLY if methyl is the last group , it is not specifically shown and Hydrogen atoms [1° or 2° or 3° ] are not even shown anywhere..
a solid line represents the attached atom lies above the plane, while a dashed wedge represents atoms below the plane