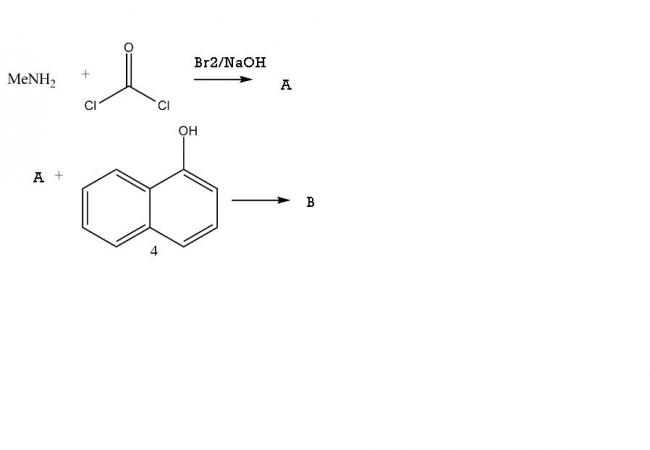
Bhopal Gas tragedy yes?
A is methyl isocyanate. B is carbaryl.
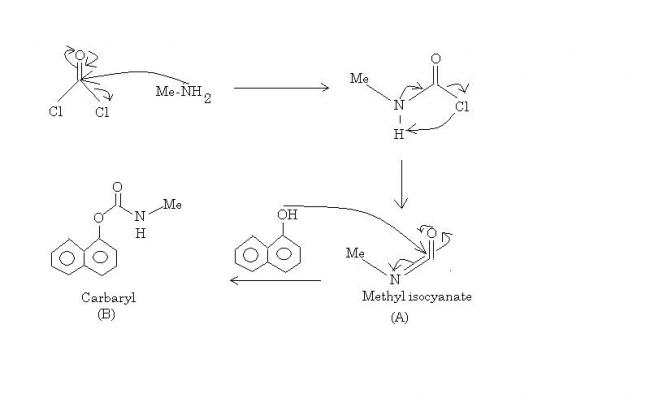
Anatomy of Disaster -:
I : Nucleophilic addition of N to CO of phosgene(which itself is a poisonous gas), or rather a direct nucleophilic substitution as the end product would be the same.
II : The intermediate so formed is called MCC. Via resonance, nitrogen forms a pi bond with C and that dislodges the last Cl. On the way it takes up the positively charged nitrogen's proton, relieving it of charge. So MCC has degenerated to methyl isocyanate(A) and one mol gaseous HCl.
III : 1-napthol nucleophilically adds to CO of methyl isocyanate. The C=O bond is reformed and the C=N bond degenerates to C-N-. Now an intramolecular acid base reaction takes place in which positively charged oxygen loses its proton to this nitrogen.
And voila. We have carbaryl(B).
Since you have mentioned basic hydrolysis, methyl isocyanate is not converted to methyl amine. That requires acidic medium.