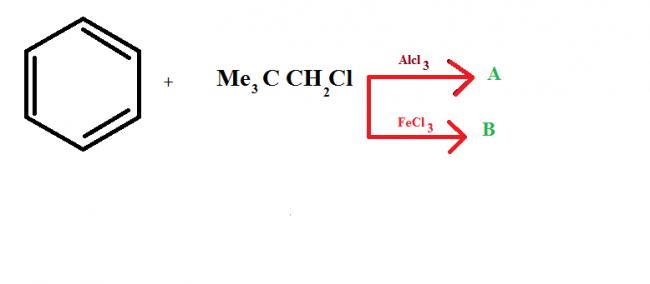
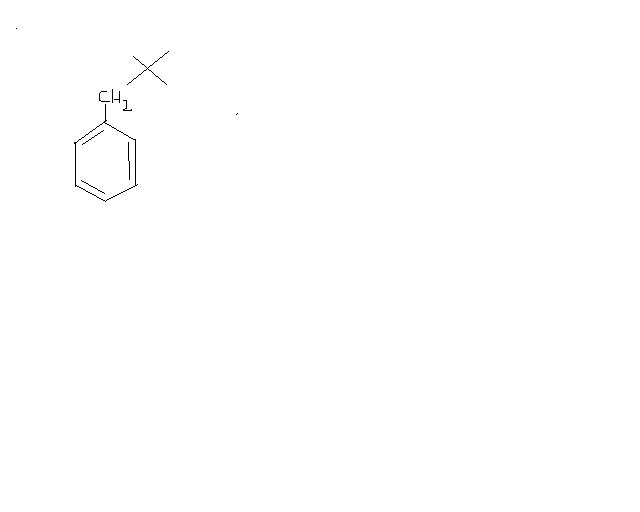
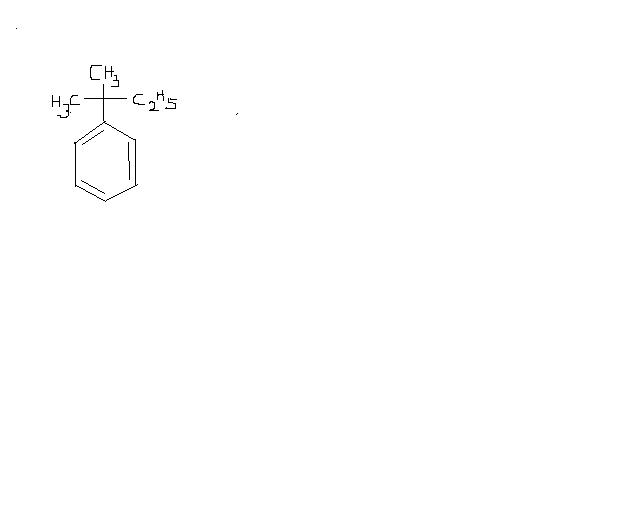
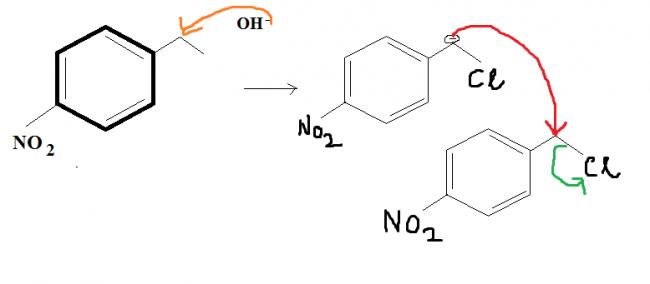
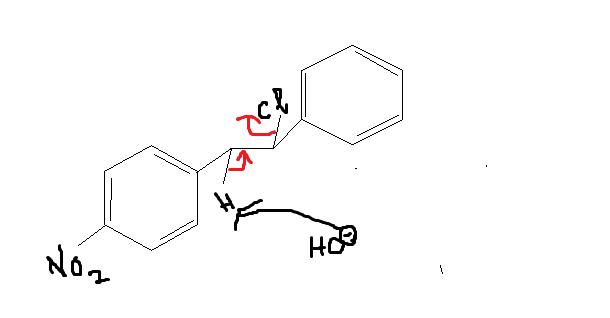
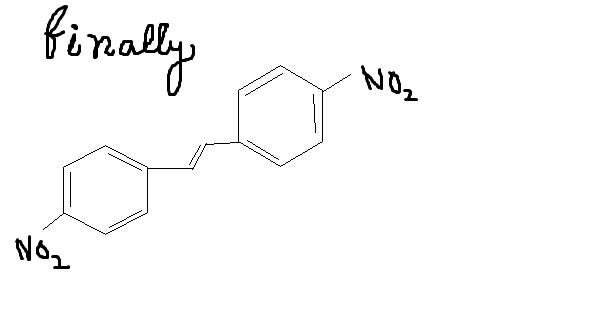
this is because of the fact that in
FeCl3, no carbocation is formed because of partial bond breakage...
whereas in AlCl3, carbocation is formed.. as AlCl3 is very much polarised.. causind the bond to break fully.. :)
14 Answers
I've kinda forgotten everything so I can't comment on both questions :/...however Joydoot's reasoning seems to be perfectly fine to me.
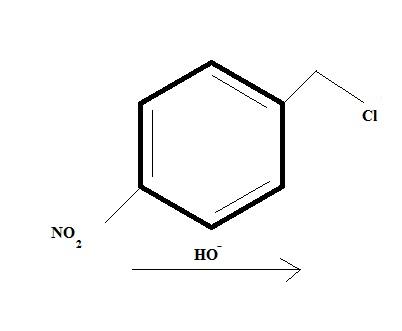
And the first one seems like a regular SN2 reaction but it's more than what meets the eye.
This awesome work doesnot belong to me of course (except the presentation)
@ shubhodip why not a direct SN2 of Cl by OH- in the first place itself ?
That (Qwerty's) will be one product....and this is another product governed by intermolecular SN2. Who'da thought...

If shubhodip's product is major, he has forgotten to write some vital reaction conditions like conc of substrate and conc of base(hydroxyl acts more favourably as a base here)..
i think Cl will be replaced in 1..........
i get confused a lot among condensation and this:P