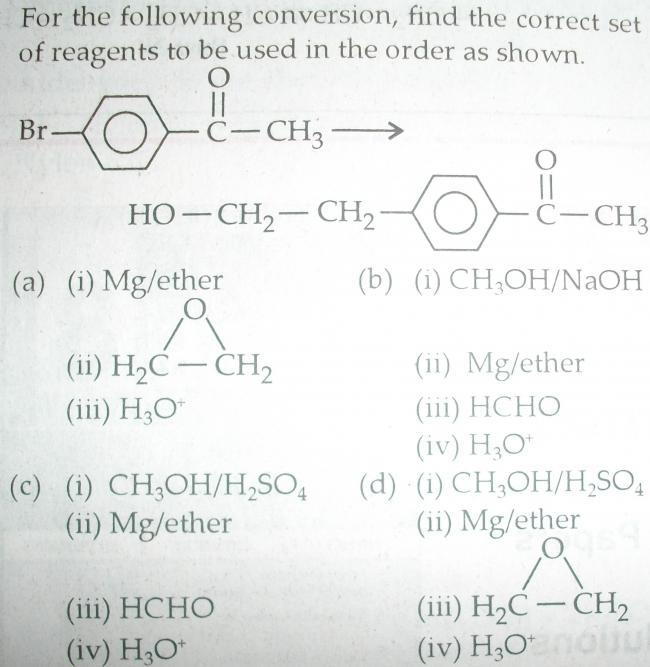
i thnk it's (a)
8 Answers
I think so too.
Edited : The other reagents involve the C=O group, which is hindered from reaction by Ph.
no answer is d
u need to block the electron withdrawing group
and alkoxy wont be electron pulling then
What's grignard addition got to do with electron withdrawing groups?
grignard reagent generates carbanion and the negative charge on carbnion is delocalised if e-withdrawing groupp is der hence not making is a strong nucleophile to attack the epoxide ring
Oh? I thought nucleophilicity is assisted by stability and basicity is assisted by localization...still seems a confusing concept.
Surely COCH3 is not that powerful a deactivator to completely destroy our yield of product in (a)...though (d) would be a better idea as per your reasoning.
anothe confusing thing in organic chemistry is
CONJUGATE Vs DIRECT ADDITION
u just cant predict the product
unless u have a lab to carry out kinetics eperiment
pritish i am also now thinking that ur reasoning is perfect
NUCLEOPHILICITY Vs BASICITY
is an old classical topic
and ya
nucleophilicity is depends on polarisabilty also more its polarised
more it will be a better nucleophile
a famous case is S- is better nucleophile than O-
taking all these things in account
i take back my views