Q2. False
Q1. III>IV>I>II (bit unsure abt position of III)
Question 1
The rate of nitration of the following aromatic compounds decreases in the order:
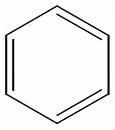 I
I
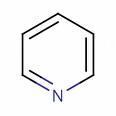 II
II
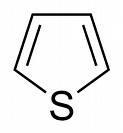 III
III
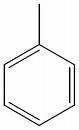 IV
IV
Question 2
True/False Type
Ph_{3}\dot{C} radicle has more number of hyperconjugation structures than those of Ph_{2}\dot{C}H
Tapas in pyridine(II), the nitrogen atom's lone pair is perpendicular to the ring system, meaning it does not delocalise over the ring. This gives the nitrogen atom deactivating character. Thus nitration is slow in pyridine.
III looks like a pyrrole analogue which has sulphur instead of nitrogen..nitration being at the highest rate here means a lone pair of sulphur delocalises over the ring(as it does in pyrrole).
@pritish agreed lone pair is not delocalized check morrison-boyd pg 1100 for thiophene thx