As it is a C-C single bond, in Q2, rotation will take place to make H anti to the leaving N+Me3 group. Product will be C(Ph wedge)(CH3 dashed) = C(CH3 wedged) (H dashed)
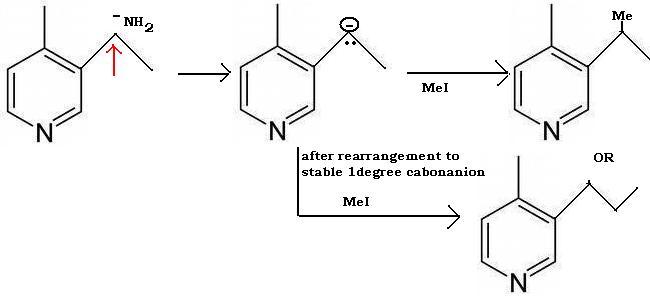
Question 1 --------NaNH2,NH3------->-----CH3I------>
--------NaNH2,NH3------->-----CH3I------>
Please write steps.
----------------------------------------------------------------------------------------
Question 2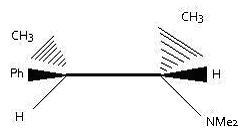 ----MeI-->---Ag2O,H2O---->---\Delta------>
----MeI-->---Ag2O,H2O---->---\Delta------>
Please write steps.
------------------------------------------------------------------------------------
Question 3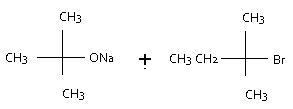 ---75.C---->----(CH3)3COH---->
---75.C---->----(CH3)3COH---->
TRUE/FALSE Type:
Major product is Less Substituted alkene and Minor Product is more substituted alkene.
-
UP 0 DOWN 0 0 13

13 Answers
Tapas in Q2 where is the nitrogen group? If it's supposed to be Hoffmann elimination..
In Q3, both substrate and base are bulky, and temperature also suits elimination. So ans is an alkene?
TRUE...base is bulky..HOFFMAN KI JAI LOL :D
See CH3I is simple. Nitrogen is nucleophilic as long as it has its lone pair, and it will substitute CH3I in an SN2 reaction. If the nitrogen atom does not have any protons to lose (to regain its lone pair) it becomes positively charged, that is, a quarternary nitrogen. This fact is exploited because a quarternary nitrogen is a good leaving group. Now usual elimination takes place, E2 style(antiperiplanar) but without Zaitsev Rule.
Q1 Answer: 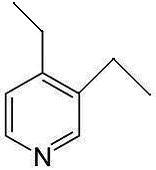
Ankur u 'seem' to be wrong
Q2: SOLVED (ans is correct)- thx pritish and govind
Q3 Answer: FALSE. Justify???
Tapas I meant for normal Hoffmann elimination...Elimination cannot take place in pyridine obviously.
Yes Tapas thats the least substituted alkene I thought of...bulky bases produce such namuneys(samples). What's wrong with that?