no the ring shift wont take place..........according to BRET's rule
bridghead C par sp2 hybridisation varjit hai!!
the conditions for a good leaving group are given as
1. It should polarize the bond which connects it to the carbon so that the
carbon has some positive charge, making it more reactive towards the
nucleophile; i.e., it should be electron withdrawing.
2. It should want to leave with a pair of electrons; i.e., it should be a stable
"anion" (not all leaving groups are anionic), which means it is unlikely to be a
strong base.
3. It should be polarizable to distribute charge in the transition state
the point 1. and 2. seem to confuse me.
going by the point 1.
a more electronegative group would polarize the carbon atom better than a less electronegative group so acc. to this, leaving group ability order
-F > -Cl > -Br > -I
but going by point 2.
we know that a larger anion is polarized more than a smaller one and so acc. to point 2. ,a larger ion must be better ,so leaving group ability would be
-F < -Cl < -Br < -I
both orders are different .plzz explain which is the right interception out of two
leaving
-
UP 0 DOWN 0 0 12

12 Answers
You have to take into account all the factors. The size of the iodine atom increases the C-I bond length, thereby decreasing the bond strength(simple chemical bonding theory). This makes it easier to break off with a pair of electrons. Fluorine is the smallest halogen, and though it is highly electron withdrawing, factor 2 and 3 work in favour of iodine rather than fluorine.
Iodine's large size allows it to delocalize the anionic charge while fluorine's does not.
ok so second order is the rihgt one
thnx sir
two more ques:
1)find the products formed in the reaction below and assign major and minor ones :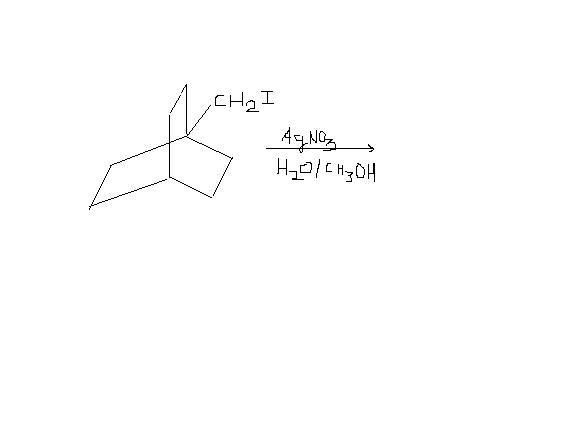
2)find the substitution product of reaction of 1-bromo-cyclohexane with CH3OH
1) First silver has a great affinity for halide ions. It will pick up I- irrespective of primary carbocation formation.
Second, rearranged carbocation(ring shift waala) will not be stable. This is because cation charge will occur at bridgehead carbon, which cannot be sp2 in nature(as it is strained). So the major product will simply be [Ring]-CH2OH and the minor one will be the rearranged one.
2) Elimination product will be slightly more favourable...but if you go by substitution, product will be an ether.
1) but the ring shift will make a 2° carbocation which is much more stable than previous 1° carbocation
Ring shift will take place but very less %age of product will be obtained through that intermediate! That is why there will be a major and a minor product. 90% will be the non-rearranged one..
bredt's rule prohibits the double bond on the bridgehead carbon because it results into a trans-ring which is not stable for rings with less than 8 carbon atoms but i m not talking about double bond formation.After the carbocation formation , there would be no double bond .The resulting product would look like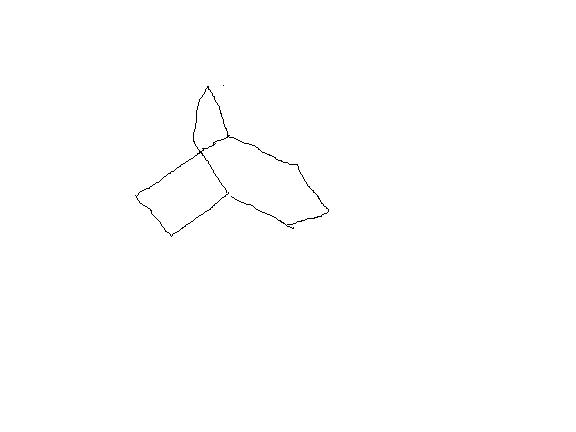
which has no double bond on bridge head carbon, so no trans form will be there.
I'm not talking about double bond formation either. But you seem to forget that the carbons involved in double bonds are planar, sp2 hybridised. That's what I'm talking about. Carbocations are planar, sp2 hybridised as well. Does that ring a bell now?
Or look at it this way. Why does the double bond not form? Because the ring strain doesn't allow the planarity. The intermediate which leads to the planar double bond is itself planar(a transition state or a cation intermediate).