pls explain mech of hydrolysis and xplain ur reasoning .............
pls yaar ......
1.explain y para nitro benzoic acid is weaker acid than ortho nitro ...... acid
2.xplain y alpha methyl acetyl acetone undergoes lesser enolisation than acetyl acetone
3.pls explain wat exactly meso means ....[i know this 1 is stupid but i havnt got it clearly]
pls explain mech of hydrolysis and xplain ur reasoning .............
pls yaar ......
para nitro benzoic acid is weaker in acidity than o-nitro benzoic acid because the nitro group in ortho position causes steric inhibition around the OH group , thereby facilitating easy breakage of O-H bond !!!! (infact, in general cases the acidity is higher for the ortho derivative..whatever the substituent at ortho position be)
for the question on hydrolysis :
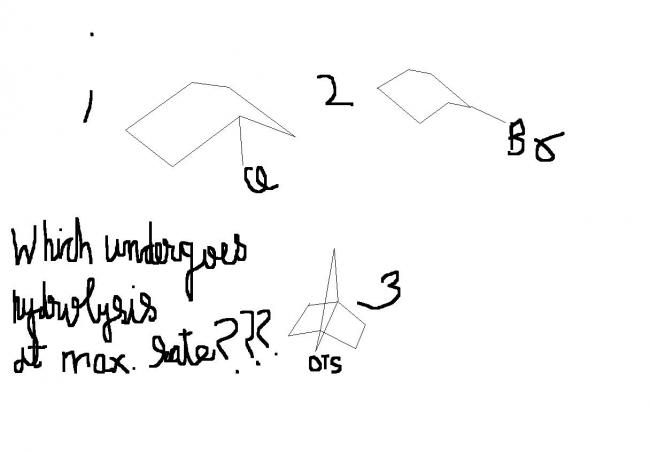
....(2) will hydrolyze the best because the leaving group is not at the bridgehead position as it is in the other two.....leaving groups don't matter here....what matters is the place from where they have to be extracted !!!
why do you want only α-hydrogens ???[2]
learn for all hydrogens
hopefully this helps
http://targetiit.com/iit_jee_forum/posts/acidity_5122.html
shrman : Can u pl indicate exactly wich all post Questn nos are still unsolved or are ur DoubtSS??
3 mein nahi hoga ..
1 and 2 mein possible hai .. but pata nahi ki axial or equatorial position is more easily hydrolysed !
Electron Withdrawing groups (groups showing -I and -R effects) increase acidity by making the C-H bond more polar. Hence if a carbon atom is attached to EWG, then the hydrogen attached to that carbon becomes acidic.
mujhe lagta hain ki 3 mein kabhie nahi hoga ..kyun ki waha ka cation kabhie planar nahin ban sakta!!
a.how to identify acidic[like how acidic they are] alpha hydrogens????????????????
b.what is bayers test???????????????????????
Q3. meso means a compound which is internally racemised. It is an optically inactive compound. One part of the compound rotates plane polarised light by some degree say @ (clockwise) while another part rotates by same @ anticlockwise hence net result --> optically inactive due to no rotation... It is usually bcz of plane of symmetry
btw nice 2 see u doing chem [3]
helpppppp!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
pls explain mechanism of hydrolysis .....................
reason:(i havent not understood yet!!) Stearic factor!!
There is also ortho effect in basicity of aniline..
if there is any group substituted at the ortho position of a benzoic acid it will be more acidic than para substituted..or not substituted benzoic acid!!
srinath pls xplain otho effect ...........
@asish seriously a pain na ... organic but i have to develop a taste for it .........
1)
in o-nitro benzoic acid, there is a double blow.......
(i) electron withdrawing effect of NO2
(ii) Intramolecular hydrogen bonding
while (i) weakens O-H bond, (ii) pulls the H further apart from O
So o-nitro benzoic acid is a stronger acid