5<3<1<2<4 ....
arrange the following free radicals in increasing order of stability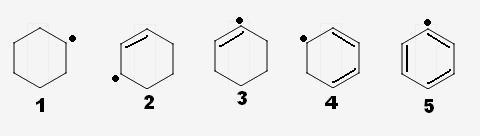
-
UP 0 DOWN 0 1 4

4 Answers
Avik
·2010-01-17 07:57:09
Dekho, 4 is the most stable, followed immediately by 2 ...as there is possibility of resonance of the free radical with the = bonds (allylic radicals).
5 - is least stable, as the aromaticity of benzene is being hindered.
Only, 1, 3 left.... So, here a secondary free radical is stabler than a secondary vinylic free radical => 1 stabler than 3.
Hence, the order.