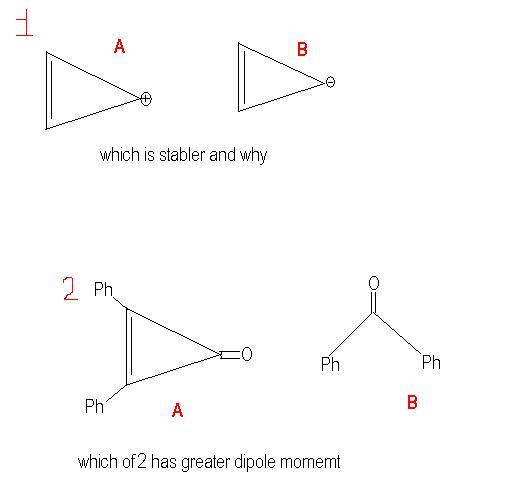
1: A
reason: pi bond is electron rich hence there will be slight repulsion in case B
11 Answers
ya ,fist one iz mor stable due to aromaticity,and second one iz antiaromatic
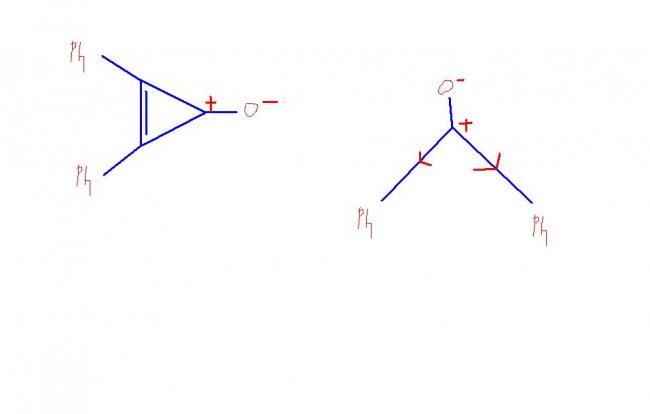
A BECAUSE IT IS MORE POLAR ...... AND THE COMPOUND IS STABILISED BY RESONANCE
IN B PHENYL GROUPS TRY TO DESTABILISE THE POSITIVE CHARGE .......WILL BE LESS POLAR .................
AND HENCE DIPOLE MOMENT
in second question.
Polarise the bond C=O by C+ -- O- ... ull see that the cyclopropyl ring becomes aromatic.. hence the ionised form is more stable .. hence the dipole moment is higher as the charge is complete positive charge
just another dbt for me in second qn,
in structure A the sp 2 hybridised carbon is strained na,but we din have this in B.Though resonance structure will give us idea,but here we have two effects which superimpose each other,so which have more impact,resonance or bondangles.