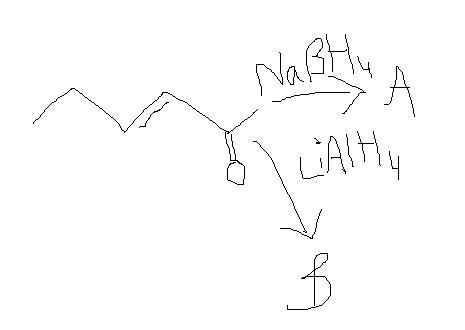
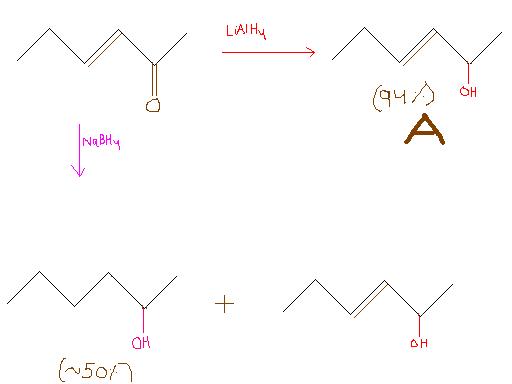
b555 se the pic above.NaBH4 does 50% times reduce double bond..
39 Answers
H2/Pt.................reduces dowuble bond....carbonyl gruop
NaBH4 ......carbonyl gruop
LiALH4 ..............carbonyl group
Oh..!!..really??....coz in Arihant and other JEE buks, it's written wat i hav written[7]
Then it's really a matter of concern coz in our classes we r taught that it doesn't reduce C=C
so what is the final say?
can some one come up with a summary of what reduces what?
According to me,
H2/Pt reduces evrything except COOH
NaBH4 reduces ald, ketone n acid chlorides
LiAlH4 reduces evrything except C=C
I've found this in many books and also on the net and even in my class notes. So, i wud stick to it.
just one thing Aditya
I found this reaction in BT material
C6H5-CH=CH-CHO→(LiAlH4) C6H5CH2CH2-OH
It says in general (Li...) is unable to reduce double bond in conjugation with CO group However the double bond is reduced when a phenyl group is attached to the β carbon
yup even i read tht LiAlH4 is an awesum reducing agent but sucks on = bond............ it cannot reduce = bond....for sure
Li Al H 4 cannot reduce c = c bond. It can reduce only polar pie bonds. But Li Al H4 can reduce c = c bonds only when the double bond is in conjugation with the benzene ring.
LiAlH4 reduces both double bond and carbonyl group
whereas NaBH4 reduces only carbonyl group(weak reducing agent)
hmm...
but Sir taught in A = is reduced when conjugation is there.
and in fitg package it say only >=O in both cases.
Hey i got something
http://pubs.acs.org/doi/abs/10.1021/jo00829a039
It says in A both(= and >=O) 50-50%
CONJUGATN wid BENZENE
Ph-C=C-C=O
thn wid NaBH4 it gives
Ph-C-C-C-OH
as LiAlH4 is strong reducing agent doesn't the double bond also get reduced?
can u please tell me why?
also once show mechanism how it reduces others........