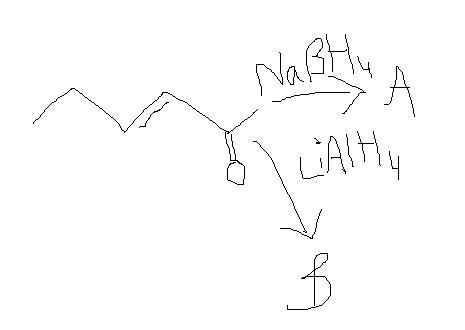
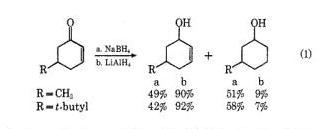
Both wil reduce only =O,so A and B are same!
39 Answers
@tapan....
wrt post #7
Ph--CH=CH--CH=O ----- LiAlH4 -------> (fatal for C=C) Ph--CH2--CH2--CH2--OH
BUT
Ph--CH=CH--CH=O ----- NaBH4 -------> (C=C is not affected) Ph--CH=CH--CH2--OH
Conjugated system
----- LiAlH4 ------>
R--CH=CH--CH=O R--CH=CH--CH2--OH (C=C is not affected)
----- NaBH4 ------>
BUT
----- LiAlH4 ------>
R--CH=CH--C--CH3 R-CH2--CH2--CH--CH3 (~100%)
|| ----- NaBH4 ------> |
O OH
see LiAlH4 is an hardnuclophile, so it attacks on more nuclophilic site, so it will attack first on c=o and if taken in excess it will reduce double bond also,
and NaBH4 is a soft nuclophile so it will attack on double bond only and
well, LIAlH4 and NaBH4 never reduce alkenes or alkynes, because they reduce by giving H- ion, which can never approach double or triple bond