oh k..yeah! i jus forgot Mr. Demanjov, he cud b an option!
WAT ARE THE POSSIBLE METHODS OF convertin HOMOGENOUS N CARBON ATOMed RING TO (N+1) CARBON ATOMed RING???
-
UP 0 DOWN 0 0 38

38 Answers
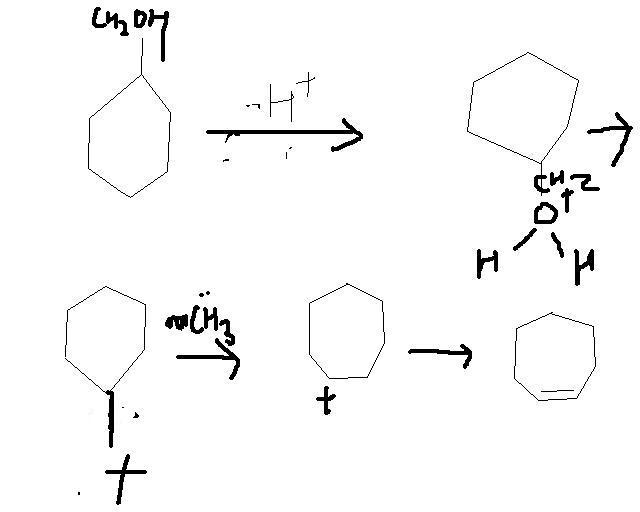
OH... RING EXPANTION......... IT CUD BE DONE WIT H2SO4 ............ TAKE FOR EXAMPLE CYCLOHEXANE WITH OH ATTACHED TO IT ..... NOW IN PRESENCE OF HEAT AND ACID IT UNDERGOES DEHYDRATION....... SEE MECHANISM IT INVOLVES SAYTSEF THING[3]
ye tumne kaise kiya? dont mind but i did not understand. u know my organic na
im not very sure abt. it!!!!! experts pls check it up....... nish bhiyya madat keejie!!!!!!!
iitimcomin..
I dont think this will be the major product.
there is hydride/methyl/any shift to give a more stable carbocation...
Here the shift gives less stability.. because the 7 membered ring will be less stable.
The better shift would have been a hydride shift to give a more stable 3 degree carbocation.
I see that as the major product.
Infact this wont even be a minor product as it seems to me..
In principle what you have done seems correct.. but do you see why this will not work!?4
but bhyya the carbocation becomes 2° from 1° ................wont thet bring stability??????