reply!!!!!!!!!
are both alkaline and acidified potassium permanganate strong oxidizing agents?
-
UP 0 DOWN 0 0 9

9 Answers
http://www.chemguide.co.uk/organicprops/alkenes/kmno4.html
nope sirrr!!!!
alkaline potassium permanganate is a strong ox agent
but in acidic medium itis reduced to Mn2+ hence becoming a strong reducing agent>>>>>
but NCERT says acidic postassium permanganate is strong oxidizing agent![3]
oye bhaskar
if gettin reduced then v say tht it is a reducing agent????
check it man ...!!!
wkt
redn is gain of electrons
and oxdn is loss of electrons
hope this suffices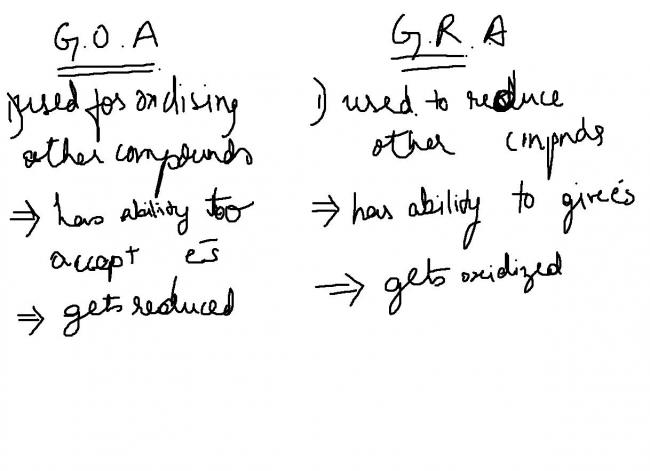
Well i answered from my own concept !!!!!! so a human error is always likely to happen ....
i'll be checkin out with wikipedia for the ans.....
hey john i got it !!!!!
WIKI says that KMnO4 in the presence of sulfuric acid gives release to some ozone which further acts as an oxidising agent.....
6 KMnO4(aq) + 9 H2SO4(aq) → 6 MnSO4(aq) + 3 K2SO4(aq) + 9 H2O(l) + 5 O3(g)
happy!!!!