thnx...mani bhai....wo toh pata hai......
but my ques is why para to Cl in both cases ; why not ortho ???
btw...in post #43 of the link that u gave ....although F,Cl,Br are weakly deactivating,they are o-,p- directing not m- directing
Pls explain why major nitration pdt. is para to Cl in both the cases given below.
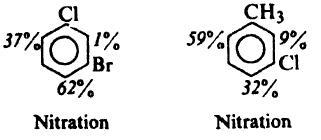
#43 may this help
http://targetiit.com/iit_jee_forum/posts/2/important_information_2774.html
thnx...mani bhai....wo toh pata hai......
but my ques is why para to Cl in both cases ; why not ortho ???
btw...in post #43 of the link that u gave ....although F,Cl,Br are weakly deactivating,they are o-,p- directing not m- directing
1st one
both are op directing and it is para to Cl because of the better inductive effect of Cl
In the second case it is para to Cl due to reinforcement...
CH3 has a greater +I effect as dat site and also -I effect of Cl is less there....
And both of them r o-p directing!!!
2nd one i think
stearic factors wont allow ortho to Cl
wrong see the corrected version below
arey subash....
on the contrary, CH3 is bulkier than Cl...so steric factor can't be the case!
sorry
here is a better explanation i think it is rite
methyl group is activating whereas Cl is deactivating
so it is decided by methyl and out of ortho and para positons of methyl group ortho on the left side is preffered because of least stearic hindrance
Yes...nice explanation!!
But one doubt...methyl group prefers both ortho n para equally...
So more +I effect on the ortho position shud b a better reason na?