why did iitcoming delete the post ??
33 Answers
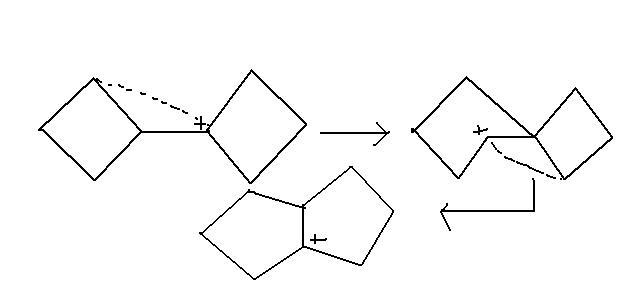
@sri...see this link.... similar kind of rearrangement..
http://targetiit.com/iit_jee_forum/posts/urgent_organic_4864.html
q2,3>1 can be expalined as in 3 its a secondary in 1 its a primary carbocation ....
then will it be spiro[4,3]octane and why wont there be bicyclo?
@ iitcoming is that the only thing ??/
@ asish .
please give me the steps thru which te yur transformation proceeds
then what will the answer be of the above one ? will it stop at the step before my final answer?
@ asish .
yes
@ sriraghav
it's pinacol-pinacolone rearrangement . not this one. this's magner weermein .
the stabilising factor is not there.
@ asish . what are your answers ?? do tell them and give your mechanism
these are quite easy . u should be able to do them
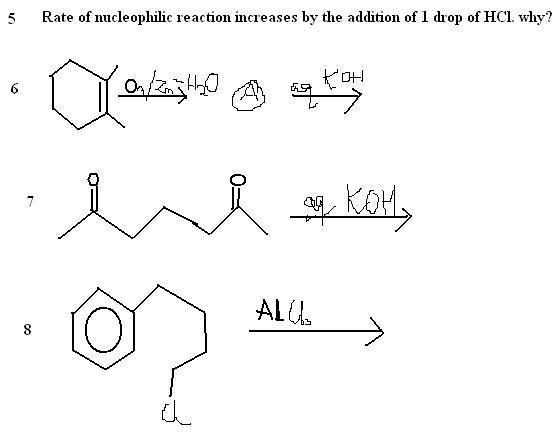
Q6. and Q7 are the same basically but i dont kno whether the 5 memberd ring structure or seven memberd ring structure will be major. According Morrison Boyd.. the ring strain is the same in cyclopentane as well as cycloheptane .. So, i dont know which one will be major
In Q8. I dunno whether first ring expansion will occur to give cyclohexane or whether first hydiride shift to 2° carbocation and then ring expansion to cyclopentane like structur.. Acc to our sir it will be the second one but in a buk i found answer first one.
Q9. Q10.. ive no idea
yes 6 , 7 are the samt. just think a bit more. you're near . :) just a lil bit
8) wat rnig expansion ??? dint get you dear . wat reaction do u think this is ????
name it
9) and 10) will give u another two days try . it's not very difficult .
one Q . have you studied alkenes and alkynes ?? or you're only solving them without proper theory study ? only asking nothing wrong meant
first one, second one is fine
third one I think you migh t be there but nomenclatuer might not be right . just check whether you're using right nomen . give structure
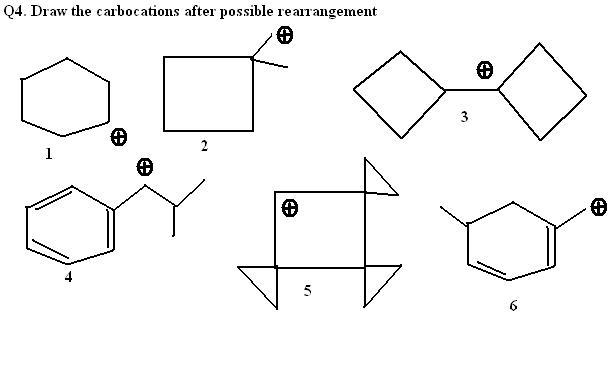
i think:
1. nothing
2. ring expansion
3. sumthing like bicyclo[3,3,0]octane
rest i dont know
DBE=(2a+2-b+d)/2
where
a=no. of C atoms
b=no. of monovalent atoms (H, Cl, Br...)
d=no. of trivalent atoms (N,P...)
O,S does no play role here
so for C5H10
it is (2*5+2-10)/2=1
its not that much important
it actually tells the the degree of unsaturation in the compound
like if the compound is C5H10 then DBE is 1
that means the compound is monocyclic or has one double bond
which you can tell directly seeing the compound
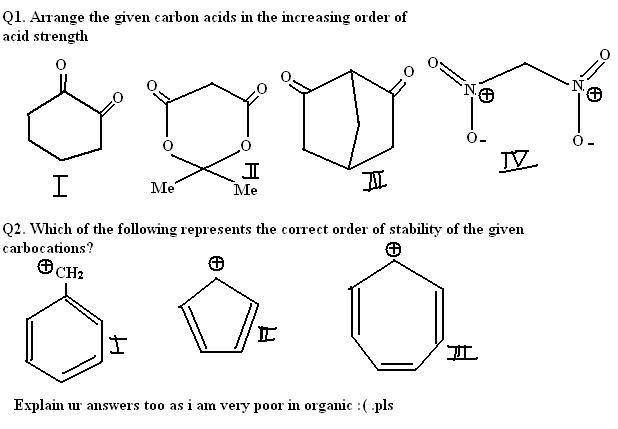
2nd explaination for acidic char. b/w 1st and 2nd
due to presence on methyl group in 2nd which is highly EWG,acidity increases
3 will have the least acidity becos the C=O gp is present ortho wrt to the alkyl gp. so it deactivates the acidic character for the molecule.
between I,II and IV
the nitro gp is a strong electron withdrawing gp so it increasses the acidic character for the molecule
so IV th will have the most acidic character.
between I and II
II is said to be more acidic becos the oxygen atom which have a power to attract the electron towards itself makes the carbon atom in the C=O bond to have more partial positive charge compared to I so it is more acidic