it's concerted try yourself and post da . this hint should suffice. try.
sry I'm very lazy :( .
now that we know that Hydroboration oxdn doesn't cater for steric hinderanxe her's a soln of atest of this site Q1 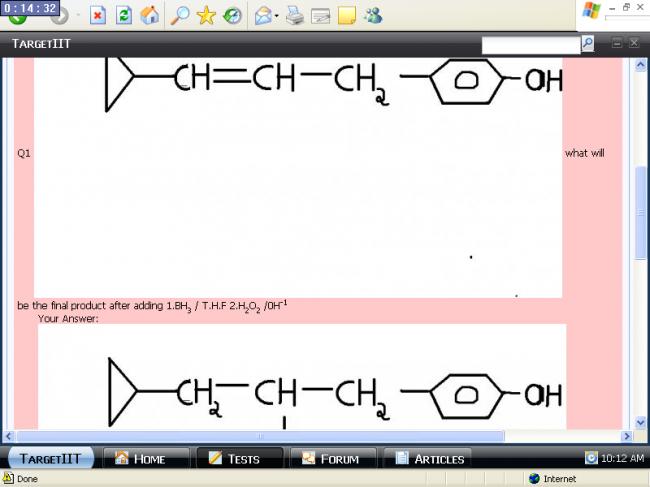 what will be the final product after adding 1.BH3 / T.H.F 2.H2O2 /0H-1Your Answer:
what will be the final product after adding 1.BH3 / T.H.F 2.H2O2 /0H-1Your Answer: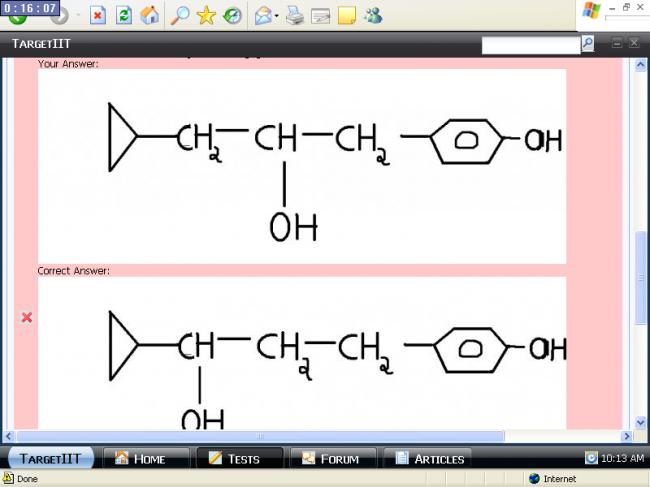 Correct Answer: Score: -5 Explaination : due to stearic hindrance BH2 will approach from less hindered site and OH will be attached there..!!
Correct Answer: Score: -5 Explaination : due to stearic hindrance BH2 will approach from less hindered site and OH will be attached there..!!
What do u say know!!!!
it's concerted try yourself and post da . this hint should suffice. try.
sry I'm very lazy :( .
look boron is more electropositive than hydrogen so B having some positive charge density attacks on the pie electron density and makes a co-ordinate bond then elongated bond of boron and hydrogen attacks the carbonium ion in this case rearrange ment products are not possible because h can attack on alpha carbon atom only as boron hydrogen bond is not broken
in this process the boron atom will adds to the carbon with least steric hinderance
the ans will be 2 methyl cyclo pentanol.can u guess the reason from the ans from this
ok guys please give the mech for hydroboration of 1 methyl penetene.please ans this question. i think it may give idea abt the mech
thank u gagar sn nishant bhaiya for ur views but i still can't understand what feenyx_21 wants to deduce
thats very true feenyx_21 but idid'nt get what u want to say about the question
Hey Guys..
according to the mecahnism of Hydro-Boration oxidation the oh- adds along the double bonded carbon atom which is less substituted..You may assume that it s similar to anti markonikoff's addition in halides..
Ex:- on hydro boration oxidation gives
on hydro boration oxidation gives

and not
,,,,,,,,,,,,,,,,
yes gaurav and abhsiek... there is a mechanism in place..
whenever users point out errors, we do fix them...
we must have been pointed out a lot of errors by our users.. we always make sure that we fix those.. :)
i think there must be a provision of rechecking in case of wrong solution and these things needs to be discussed