okie.. give me sometime.. i need to think..
and den draw d resonance structures!
what is the meaning of conjugate pi bonds???? plz explain with example!
yes.. see what do u think?
it is not tough..
i will give one hint.. oke
N can do two things.. donate or accept electrons
lets try accepting a lone pair.. what will happen?
If u cant tell me i will do the first step :)
okie.. give me sometime.. i need to think..
and den draw d resonance structures!
see i am posting the resonance structures.. i hope it helps..
sorry ashish if u already did some hardwork..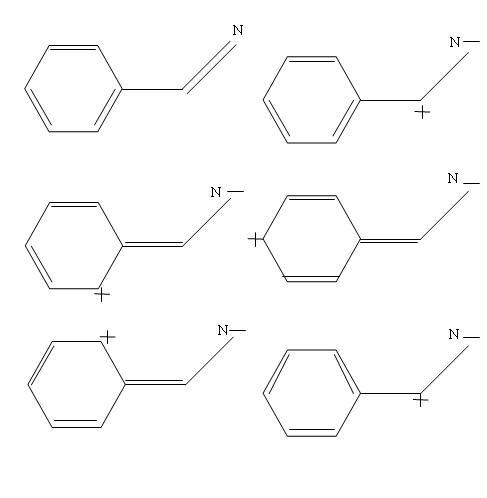
About resonance.
See the thing is that lot of atoms that can donate or accept a lone pair
Things to keep in mind
1) Resonance structures are imaginary
2) Resonance structures have the same number of electrons (There is no extra electron added or removed from the structure)
3) All resonance structures must be valid Lewis dot structures.
4) The hybridization does not change (This is very important!) sp3 hybrid carbons will remain sp3 and so on
5) The positions of atoms are the same in all structures. Ie the basic structure of sigma bonds remains the same
6) The actual structure of a molecule is an average of all resonance structures.. each having difft weightage (sometimes similar)
yeah.. but whenever i solve any problem.. i don't get where will the pi electrons will move. if there are more than 2-3 double bonds it creates confusion.
Now how to draw resonance structures..
in the question that u have given. N can both accept and donate lone pairs (Hwo do i know this (it is a fact!) also it depends on the fact that NH4+ and NH2- can both be formed)
So we can try both. it is not necessary that both will give a good structure.!
We accept the lone pair of the double bond towards the nitrogen ... so extra charge gets transferred so N becomes "N-"
the carbon atom from whom the charge was taken develops a +ve charge...
This is the first resonance structure
continued....
yeah.. but whenever i solve any problem.. i don't get where will the pi electrons will move. if there are more than 2-3 double bonds it creates confusion.
yes u are rite.. it is confusing at times.. just make sure that the structures u are making are valid and have the same basic structure (I mean the skeleton of carbon atoms)
now again in the above structure,, what we have is that the double bond of the ring gets transferred by donation of the electron from the double pair to the +ve carbon atom...
so the +ve charge comes to the benzene ring!
and the double bond gets transferrred..
I think i made a mistake of not drawing the arrows of electron movement in the resonaance structures.. (I hope u can figure the movevemnt ur self!)
so can u plz explain me the resonating structures of the given structure : -
CH2=CH-NH-CH3
i think
CH2-CH=NH-CH3
the first carbon atom with - charge and N with + charge,
, it should be like dis.. but i m not confirmed..
see it is nothing to worry much..
basically you will first guess what will happen if the electron transfers from this place to that!
then the structure that is formed should be valid :)
that is all...
now try to do the ulta thing.. N accepts an electron :)
initially N has a lone pair, when the lone pair moves from N towards the left side.there forms a double bond between CH and NH and the double bond between CH2 and Ch breaks.... so ultimately there is one unpaired electron left with N.
yes good (but incorrect) observation...
see what happens is that out of 2 electrons of a lone pair, one goes in the formation of the bond.. the other electron gets transferred to the other (neighboring) carbon atom.. so there is no unpaired electron in that sense :)
also, when the electron is shifted towards the nitrogen atom from the double bond.. see there were 2 electrons in the double bond.. (one shared by each atom) so what happens is that both these electrons come to the nitrogen atom..
So there is an extra lone pair formation.. on Nitrogen.. with ony one extra -ve charge!
what do u mean by "separated by 2 carbon atoms" 2nd bond is also separated by two carbon atoms!!!! i m sorry... but i have a lot of confusion !!!!
Conjugation is possible by means other than the presence of alternating single and double bonds. As long as each contiguous atom in a chain possesses a p-orbital, the system can be considered conjugated. For example, furan (shown at right) is a five-membered ring with two alternating double bonds and an oxygen in position 1. Oxygen has two lone pairs, one of which occupies a p-orbital on that position, thereby maintaining the conjugation of that five-membered ring. The presence of a nitrogen in the ring or groups α to the ring like a carbonyl group (C=O), an imine group (C=N), a vinyl group (C=C), or an anion will also suffice as a source of pi orbitals to maintain conjugation.
It is important to note that merely possessing alternating double and single bonds is not enough for a system to be strongly conjugated. Some cyclic hydrocarbons (such as cyclooctatetraene) do indeed possess alternating single and double bonds. Although the molecule may appear planar if one looks only at its chemical structure, it is in fact not, and typically adopts a "tub" conformation. Because the p-orbitals of the molecule do not align themselves well in this non-planar molecule, the electrons are not as easily shared between the carbon atoms. They can be still considered conjugated, but they are not considered antiaromatic (and also not aromatic; see Hückel's rule). Cyclooctatetraene would not be considered antiaromatic because it is not planar.
a Question is given that in the given molecule
CH2=CH-CH=CH-CH2-CH=CH2
identify the conjugated bonds.
How to solve this question!
CH2=CH-CH=CH-CH2-CH=CH2
see only the first and 2nd double bonds are in conjugate pair.(1stand 3rd bonds) the other double bond at theend does not act as a conjugate bond. If u know resonnace, then it is obvious that the 3rd double bond will not participate in resonance..... so only the first two will be the answer
actally i have started organic chemistry just a few days ago.. so i m facing a lot of problems in resonance ........:(
resonance is one of d relatively easy things :)
just study it easily. :)

Take the example of benzene (shown above). In a Lewis diagram, two carbons can be connected by one or two covalent bonds, but in the observed benzene molecule the carbon-carbon bond lengths are 139 pm, longer than typical C=C double bonds (133 pm) yet shorter than typical C-C single bonds (154 pm). More importantly, they are all equivalent, a fact no Lewis structure can explain. Therefore one calls the two Lewis structures canonical, contributing or resonating structures and the real molecule is considered to be their average, called a resonance hybrid. Resonance structures of the same molecule are connected with a double-headed arrow.
This form of resonance is simply a way of representing the structure graphically. It is only a notation and does not represent a real phenomenon. The individual resonance structures do not exist in reality: the molecule does not inter-convert between them. Instead, the molecule exists in a single unchanging state, intermediate between the resonance structures and only partially described by any one of them. This sharply distinguishes resonance from tautomerism. When it is said that a molecule is stabilized by resonance or that amides are less basic because the lone pair on nitrogen is involved in resonance with the carbonyl pi electron, no phenomenon is implied. What is meant is simply that the molecule behaves differently from what we expect by looking at its Lewis structure because the structure diagrammed does not represent the actual structure of the molecule. From this viewpoint, the terminology treating resonance as something that 'happens' is perhaps an unfortunate historical burden.