let me be frank to all.........i am pathetic in structure based problems.........i dont know how to solve them....i dont know where to start from............
so plzzzzzz solve this and some more questions which i will post in detail so that i could learn......plzzzzz
i hope u all will help me....
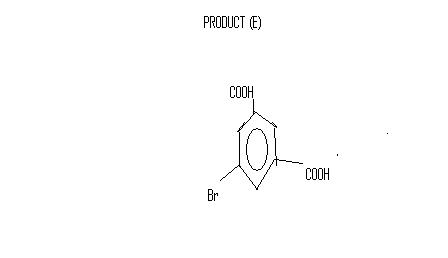
An organic compound A (C8H10) does not decolourise aqueous soln of Br2 and on treatment with Br2/FeBr3 in dark yields 2 products in principle B and C but due to steric hindrance B predominates .A on refluxing with alkaline solution of KMnO4 yields D (C8H6O4) which gives off a gas on treatment with NaHCO3.D on treatment with Br2/FeBr3 reacts very slowly to produce E(C8H5O4Br) as the only isomer.Also D on treatment with excess SOCl2 followed by work up with benzene solution of AlCl3 produces F(C14H8O2) which does not yield any gas with NaHCO3.F on heating with N2H4 produces G(C14H8N2).
Find A to G.
-
UP 0 DOWN 0 0 21

21 Answers
both u should know the common names but of simple compounds only
i did write the name b'cos making the structures is a hectic job and again the dtp sir taken a holiday today .....
one more doubt.............in jee will we be given structures or their names in options in these roadmap questions????
i will do it but after 8 pm b,cos my dtp is gone for a break whenever he returns i will post them for u
sir can u plz draw the structures...i am unable to figure them out by their names................or should i draw ?????
rxn of KMnO4 bring four oxygen means two CH3 groups in benzene
rxn of SOCl2 bringing only one benzene means only one CH3 or two CH3 in ortho position above two suggested the A and rest was easy
sir plzzzz explain too..............i am still learning how to attempt them.....[2]
A is ortho-xylene
C is 2,3 di-methyl bromo benzene
B is 3,4 di-methyl bromo benzene
D is pthallic acid
F is 9,10 anthraquinone
for other products i will give the structures in next post
well in that structure question if A is 1,2 dimethyl benzene then still para product should be prominent but in that case problem would arise in the 4th reaction where only one product is expected.
C8H10 hi hai yaar.............
mujhe kuch aur mat poochna kyunki mujhe aise ques bilkul nahin aate........jaise tum log sikhaoge .....vaise hi main karoonga....[1][1]
one question.. where will the bromine get substituted? will it substitute a methyl group or will it substitute a Hydrogen of Benzene?
no, in one product bromine would be ortho wrt to both methyl groups and in other it will be ortho wrt to one and para wrt to other
even in 1,3 dimethyl benzene.. both substituted products using Br2/Fe are identical
that is not 1,2 dimethyl benzene, its 1,3 dimethyl benzene ,i have edited that post. btw have you any idea about the F part?
saket how can 1,2 dimethyl benzene give theoretically two products with Br2/Fe but one of them is sterically hindered? Please give a diagram..
for the third reaction, D should be(1,3 benzene dicarboxylic acid) as KMnO4 oxidises the methyl groups to carboxyl groupsand the gas given out is carbondioxide.
* for E as only one product is possible so it should be the meta position where bromine should be substituted
shouldnt A be 1,3 dimethyl benzene as it a saturated compound and will not decolorize bromine water. * and i guess in second reaction the two products B and C bromine is substituted at ortho and para position out of which para predominates as the steric hindrance will be less there.