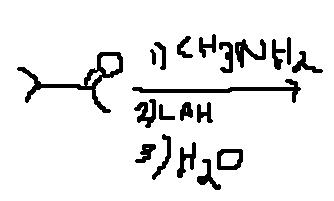
and for beckmann we need acidic mdm no ? it's migration towards electron deficient nitrogen
38 Answers
in the mechanism, removal of H2O takes place thats why i have made this shortcut of removal of water(H2O).......
can u give me the full mechanism or if it is a name reaction can u give me the name
hey u can find the reaction in NCERT in reactions of aldehyde with ammonia derivatives....
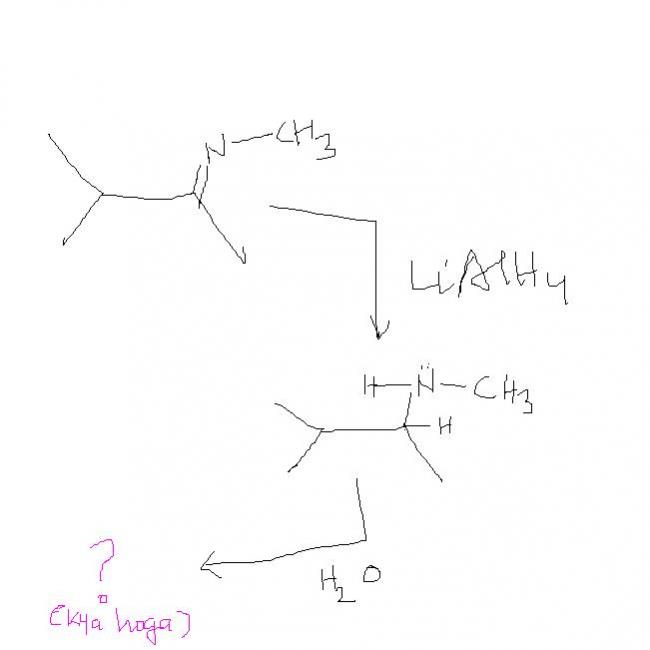
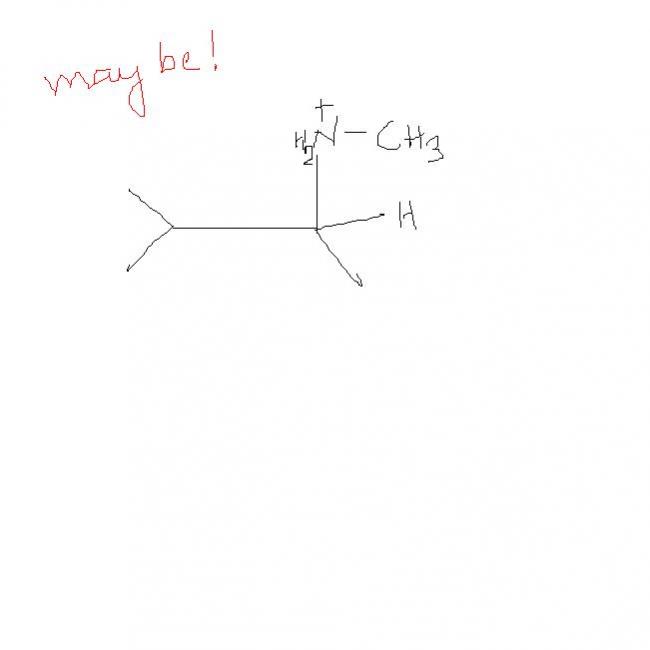
so wats the answer. ????sky has givne it only thing is she has hydrolysed without using water. that's where water comes in , with LiAlH4 we don't get sky's product but we get it on hydrlysis.
I think Sky is correct.
When ketone reacts with primary amine we get Imine and not oxime. Hence Beckman rearrangement is not possible even in the absence of LiAlH4.
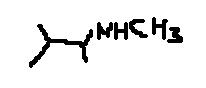
When Imine is reduced with LiAlH4 what we get is amine (in this case secondary amine. I notice that the final product has a chiral carbon
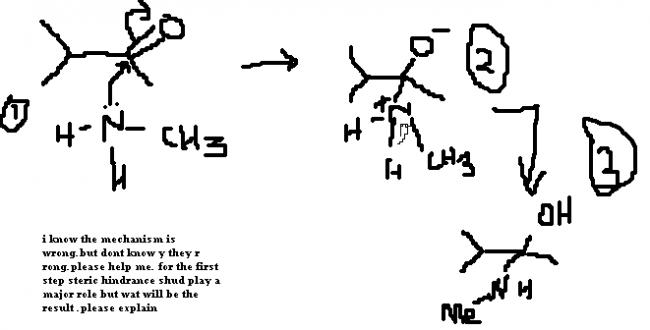
but buddy i think the first step shud be the formation of oxime...
which will get reduced in second step after rxn with LiAlH4...........
guys beckmann willn't be there...
there is something else....
@pankaj....
can u just find the use of water in this reaction.....
i will ask them n tell [3]
but thiis happens .....
arey this has some name...!
commonn reaction...
arey, i got it....
Firstly, it react with CH3NH2 to form oxime....
Which the rxn with LiAlH4 result into 2 degree aminel....
Which will then follow beckmann rearrangement with OH- ion of water.....