i included steric hinderance so i will dot the option A
well, today seems to b organic DAY!!! [4]
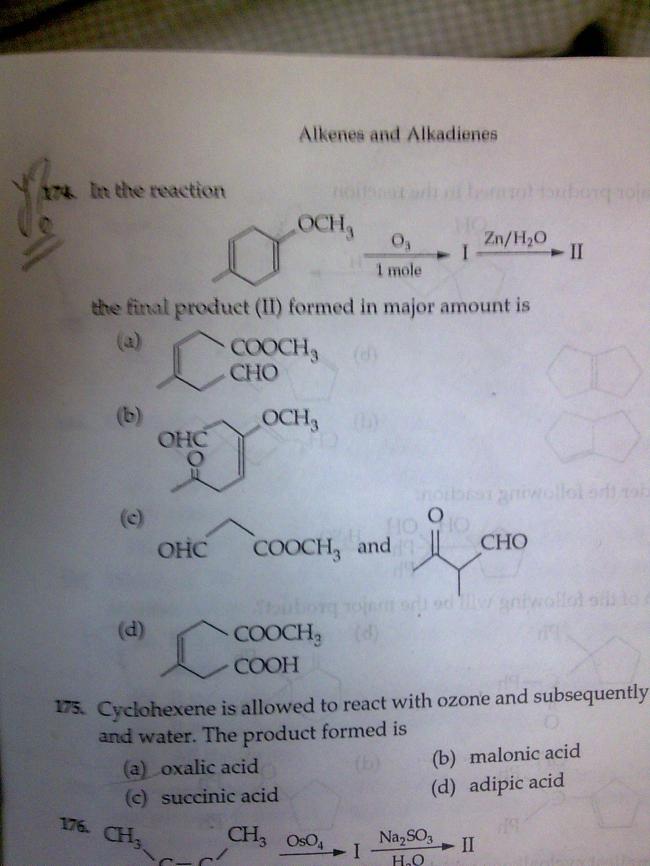
so try this one : ans is "A"
pl justify!!!
-
UP 0 DOWN 0 0 21

21 Answers
[11] [11][11][11][11][11][11][11][11]
that means ans A is correct and the mechanism in MTG publication has some flaw (mayb) (SORRY TO MR. MUKUL RAY)............
is 'sykes' quite a dependable source Sir?
@sankara.. there is equal steric hindrance at both the sites..
infact more on the right one..
Yes I think so because the rate determining step is this attack..
But I am not absolutely sure on this one.
OH K K.... THEN I BETTER BELIEVE that its O+ which attacks and not O-
thnx for the wonderful reference!!!
@priyam, sky it is the O+ attacking...
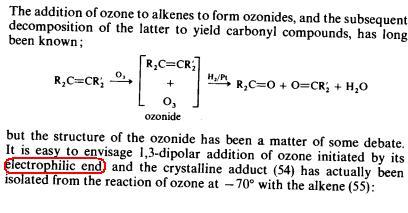
See peter sykes' thing that i posted.. It writes that the part of ozone which is electrophilic initiates the attack. So O+ is the one that attacks.
I have no doubt on peter sykes. That is one of the best books on mechanism that is available.
nahi ... double jake attacj karta hai ... O pe...
was telling naa priyam.... [saw sir's notes]
mechanism as in : "Name Reactions" by Mukul C Ray
but in this O- is attacking... [7]
it has to be (a) or (b)...
so theres 50-50 chance.. take it... make a tukka... [3]
From the above it is clear that the +ve charge of ozone will attack first..
so it will attack a electron rich center.....
+m is acting on both is distance independent but -i is distance dependent n more on right one.so right one more electrophilic thus my ans is correct.
mechanism as in : "Name Reactions" by Mukul C Ray
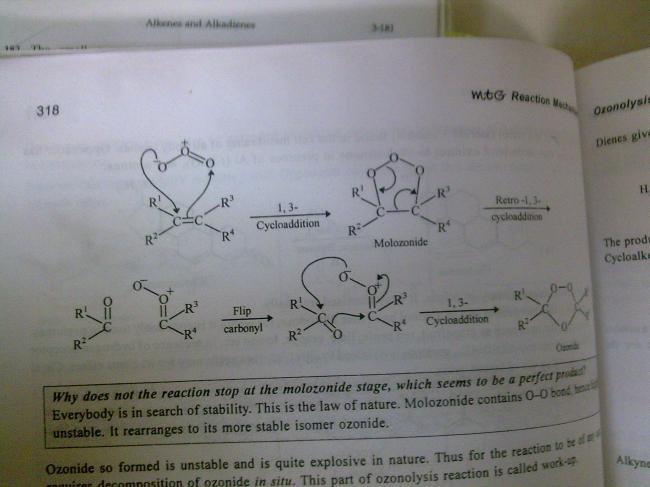
this mite aid........
but it actually contrasts the answer..........
@ priyam.. yes ur tukka is half correct..
only we have to decide which in a or b
bcos we have to see which one Ozone will attack first..
I think the "left" double bond because there is +R effect on the right one..
I think it should be b :)
well i think it shuld be (b) because O-CH3 is involved in resonance with the double bond.
I dunno i myt be wrong.
as we know dat 2moles of o3 ll be reqd for complete oxidation but we r given 1 mole so more electrophilic bond ll be attacked n it s d right one
if u need further explaination tell me i llexplain it