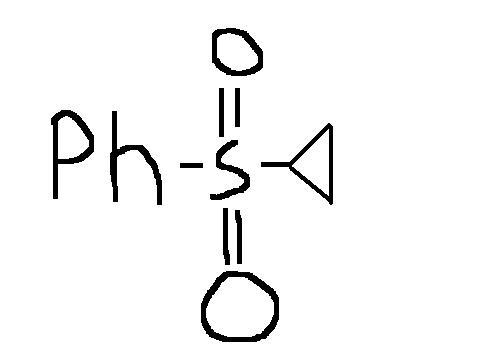
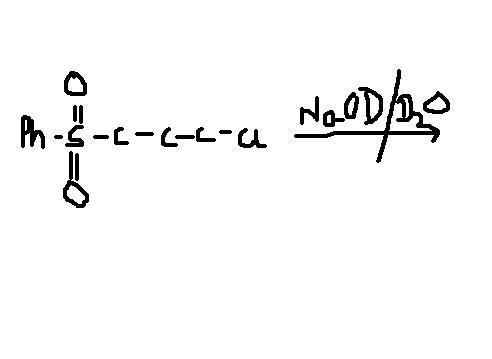yup this is one question that i also want to know the answer to...
@pritish
just replace unfulfilled bonds with hydrogen...that much is understood....
14 Answers
Is the diagram correct??? So many carbons with unfilled bonds..
On both carbon 1 and 3 there are good leaving groups. OD- can attack both by SN2..what say?
Being resonance stabilised, the left side group is a better leaver, and it is substituted. After substitution, since the C-OD and C-Cl bonds are syn periplanar, there is no scope for the formation of a ring by SNi(internal). SNi is supported when 3, 5 and 6 membered rings are formed.
thats the main problem dude the left side group is not a good leaving group....!!
I know the answer!!!!!!![3]
If I give the answer, can anyone explain the mechanism??
arshad, how is it not a good leaver? Its a resonance stabilised anion..weak base.
Nvm. got the mech.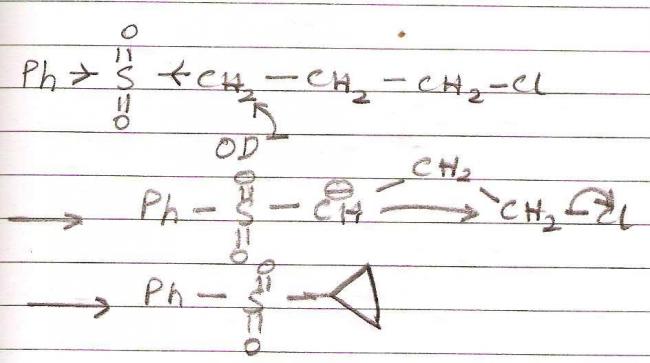
Explanation :
Due to electrophilic character of sulphur, the carbon adjacent to it becomes electron deficient as sulphur pulls electrons towards itself. Now NaOH or NaOD are strong bases. Their primary job is to abstract protons, which they will do so from the most acidic carbon.
After formation of carbanion, SNi takes place as chlorine is a good leaving group and kinetics favours the formation of a three membered ring.
This is similar to the Favorskii Rearrangement.
in the acid base reaction of OD- and the given compound ....pritish has shown the formation of carbanion ..... so for dat the carbanion has to be stable enough for d equilibrium to shift towards right ....so is the resonance stabilization of the carbanion with that grp on d LHS of d given compound one of the factors?
Actually bhai when the base is strong, the equilibrium automatically shifts far to the right and a carbanion is formed with no scope of other carbanions forming from the same substrate. It is what happens when we use bases like LDA in aldol condensation.
And ty for the cheers iota :P
Edit : it is also stabilised by sulphur's -I like effect.
yeah ...but isnt a simple carbanion a stronger base than OD- ??and here is it a resonance stabilized C - ??
btw dont scare me by using words like LDA and all ...i hav jus completed Hydrocarbons .. [3]
It is simple a ring of propane will be formed replacing the other C atom and the other product is HCl