wat are L isomers?
1)
haemoglobin is
1)an organometallic compound
2)chelate
3)conjugate gloubular protien
4)all above these
2)proteins contains
1)exclusively D isomers of amino acid
2)mostly L isomers of amino acid
3)50% of each D- and L- amino acids
4)33% of D- form and 67% of L- form of amino acids
xplain why
-
UP 0 DOWN 0 0 4

4 Answers
1) 4.
Reason: it contains Fe, and four chelate rings. N---Fe bonds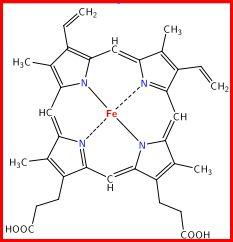
2)2 factual
ISOMERS By configuration: D- and L-
An optical isomer can be named by the spatial configuration of its atoms. The D/L system does this by relating the molecule to glyceraldehyde. Glyceraldehyde is chiral itself, and its two isomers are labeled D and L (typically typeset in small caps in published work). Certain chemical manipulations can be performed on glyceraldehyde without affecting its configuration, and its historical use for this purpose (possibly combined with its convenience as one of the smallest commonly used chiral molecules) has resulted in its use for nomenclature. In this system, compounds are named by analogy to glyceraldehyde, which, in general, produces unambiguous designations, but is easiest to see in the small biomolecules similar to glyceraldehyde. One example is the amino acid alanine, which has two optical isomers, and they are labeled according to which isomer of glyceraldehyde they come from. On the other hand, glycine, the amino acid derived from glyceraldehyde, has no optical activity, as it is not chiral (achiral). Alanine, however, is chiral.
The D/L labeling is unrelated to (+)/(−); it does not indicate which enantiomer is dextrorotatory and which is levorotatory. Rather, it says that the compound's stereochemistry is related to that of the dextrorotatory or levorotatory enantiomer of glyceraldehyde—the dextrorotatory isomer of glyceraldehyde is, in fact, the D isomer. Nine of the nineteen L-amino acids commonly found in proteins are dextrorotatory (at a wavelength of 589 nm), and D-fructose is also referred to as levulose because it is levorotatory.
A rule of thumb for determining the D/L isomeric form of an amino acid is the "CORN" rule. The groups:
COOH, R, NH2 and H (where R is a variant carbon chain)
are arranged around the chiral center carbon atom. Sighting with the hydrogen atom away from the viewer, if these groups are arranged clockwise around the carbon atom, then it is the D-form. If counter-clockwise, it is the L-form.
SOURCE:guess what?