Correct...so there won't be any haloform group leaving in this case.
why the first one can show iodoform test but not 2nd one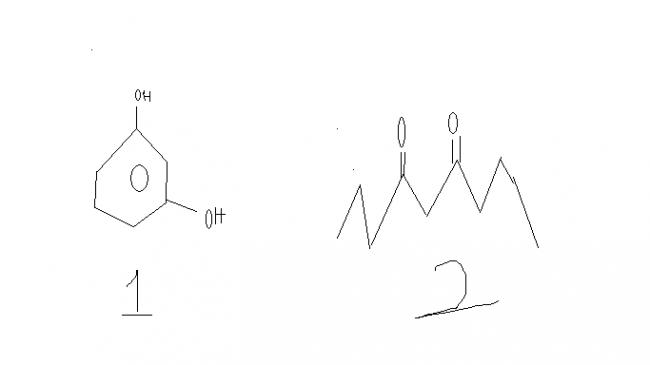
-
UP 0 DOWN 0 1 6

6 Answers
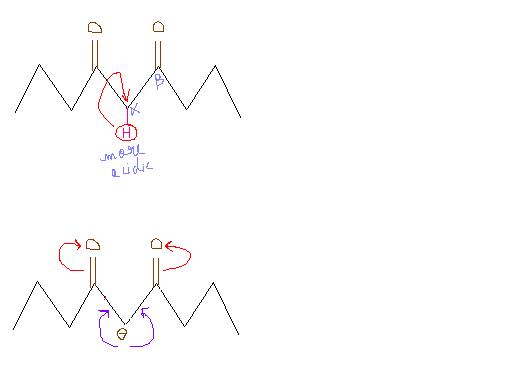
This is bcoz in the 2nd structure, the hydrogen of ketomethyl group is not abstracted in the first step of the mechanism as the alpha hydrogen on the other side is more acidic.
NOTE → The alpha Hydrogen of the methyl group shuld be more acidic. If the alpha hydrogen on the other side is more acidic, then Haloform reacn cannot takes place.
i know this fact.
as the middle carbon is more acidic so first iodisation will occur at this carbon,this is the same case as tht of the iodisation step of p dihydroxy benzene,but why only p dihydroxy benzene can give haloform test but not the second one,thts my doubt
guys see the mechanism of first one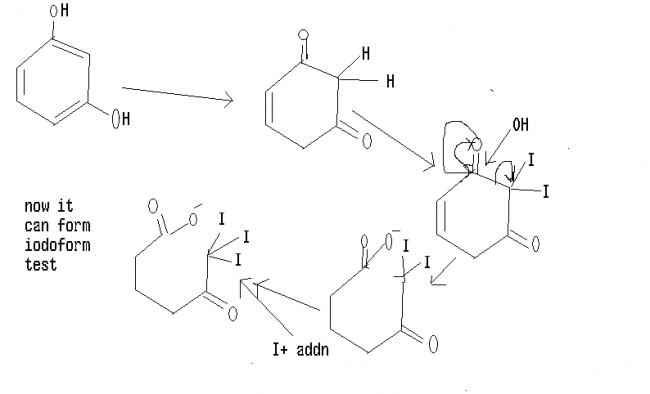
my doubt is if this can show this type of reaction then why second one can not
as p di hydroxy benzene there are two alpha hydrogen atoms present to initiate iodoform rxn,but in 2nd case no alpha hydrogen
note :
in 1.3 dihydroxy when it undergoes tautomerisation it forms a diketone whish is unstable soon react with base to undergoes furthur idoform reaction '' CH2 is acidic in nature"
but in case iof dicarbonyl compouds of 2nd one have ability to form intramolecular hydrogen bonding hence acidity of CH2 GROUP DECREASES hence it will not give iodoform test
thanks