a) will react fastest becoz alkyl group always give +i effect so electron density increases more in case of a) and not in b) c) and d) as the repulsion between electrons increases , R is present both sides...
Q→ which of the following will react fastest with H2 under catalytic hydrogenation conditions ?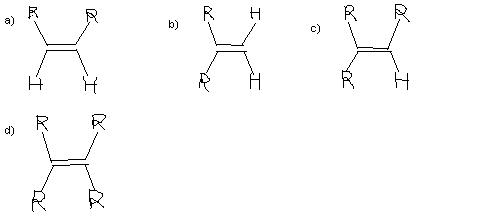
-
UP 0 DOWN 0 0 5

5 Answers
qwerty
·2010-09-23 01:31:16
a>b>c>d ...reactivity towards catalytic hydrogenation ....i.e a reacts fastest
more stable the pi bond , less is its reactivity towards catayltic hydrogenation
kajal
·2010-09-23 01:56:42
Hydrogenation of alkene is governed by the fact stearic hinderance. Smaller the alkyl-R group lesser will be the stearic hinderance.Thus in option (a) alkyl -R gp on the same side,soit react fast.
nihal raj
·2010-09-23 05:24:32