1. The base is strong. Strong unhindered bases prefer bimolecular eliminations.
2. E2 involves an anti-periplanar transition state (the leaving group and the abstractable hydrogen are anti to each other).
3. In this compound, the beta hydrogens and the leaving group are syn. E2 is not possible.
Hence no elimination product is obtained.
Only a substitution product is obtained when the compound below is treated with sodium methoxide. Explain why an elimination product is not obtained?
Compound is 1-bromo-2,6-dimethylcyclohexane
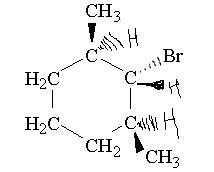
-
UP 0 DOWN 0 0 12

12 Answers
in the TS , entropy of TS is already less than that of reactants , and so reaction proceeds towards unstability ,
now anti form will be more stable than gauched or staggered ,
hence E2 eliminations always prefer anti elimination so as to decrease unstability of TS ,
and here no H is anti to Br , hence no E2
Ok so reaction will proceed via SN1 or SN2?
I think it should be SN1 because there is a sec. halide.
One more thing- @Pritish why CH3O- is a strong base. Strong compared to what? Is strong base always a good nucleophile?
I meant unhindered...not strong lol. Methyl alcohol is slightly acidic and more acidic than its straight chain counterparts. Hence its conjugate base is stable, not strong.
Your sentences always have double meanings [3]
So will it be SN1 or SN2?
poor nucleophile/weak base favours SN1
strong nucleophile/strong base favours SN2
This is what I know.
I would go for E1 as E2 elimination isn't possible. The methyl groups attached to the beta carbons cause too much hindrance to back-side attack for SN2 to happen...here the base finds it easier to abstract a proton instead.
E1 and SN1 both follow the same priority order(3 > 2 > 1), and the carbon is secondary. I would go in favor of E1 I guess.
It is given in the question that there is no elimination product obtained.
Did you mean E1 as SN1 [3]?
E1 doesn't require an anti-periplanar transition state, and it has the same priority order as SN1. So I suppose they'll go neck to neck...50-50?
Or you could reason that a stable base works better as a nucleophile, and substitution product is major.

Maybe the question actually asked about why the E2 product isn't formed, because I've done a similar question before.
We"re giving sodium methoxide here,isn"t it??
so that makes this a kinetically controlled reaction,
that is, SN2 ...