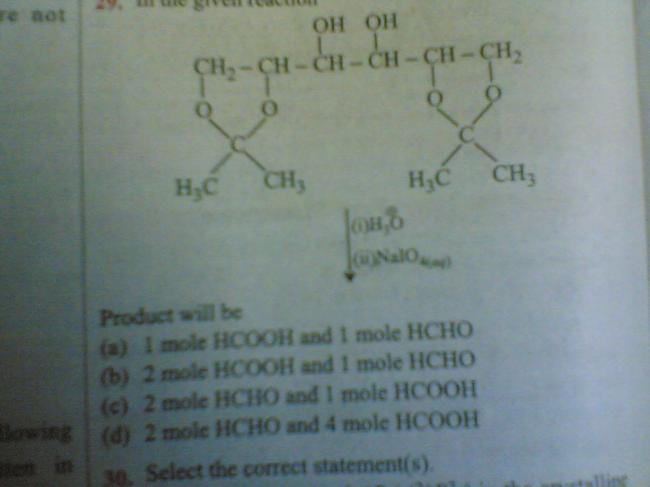
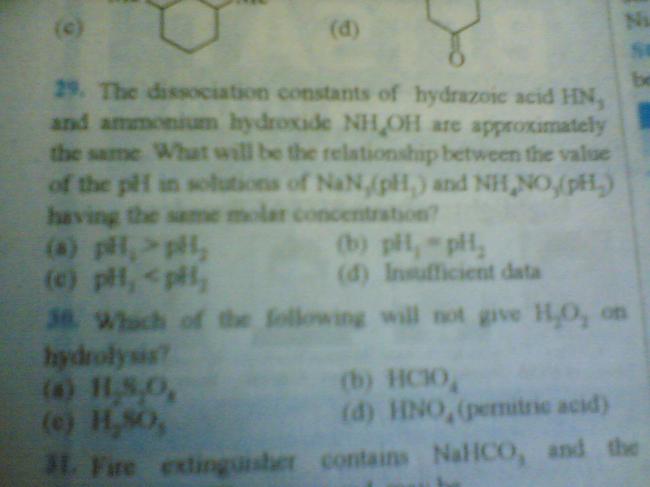guys pls help it is urgent for me.
13 Answers
1. (d)
Notice first H3O+ is used..first write product after attack of H3O+.. then it will be obvious...
abi wat is the function of NaIO4 can u pls give the full mechanism for ur correct ans..
no need for mechanism as I think it is not directly there in syllabus...
But What you have to do is...just break bonds between carbons(when both of adjacent carbon has -OH..)and add one -OH each to both... then those carbons will have two -OH ... then u know what to do when a carbon has two -OH...(ketone or aldehyde)
when a -OH bearing carbon has -OH bearing carbons on both sides then there will be two -OH added to it...
so net three -OH so acid will be formed here..
kk priyam.Can u give me abt the function of using NaIO4.Becos i have seen in many qns.
I think it is used for conversion of organic halides to carbonyls.....though my chem is weak
that was the function of NaIO4
i forgot to put up the image... here it is: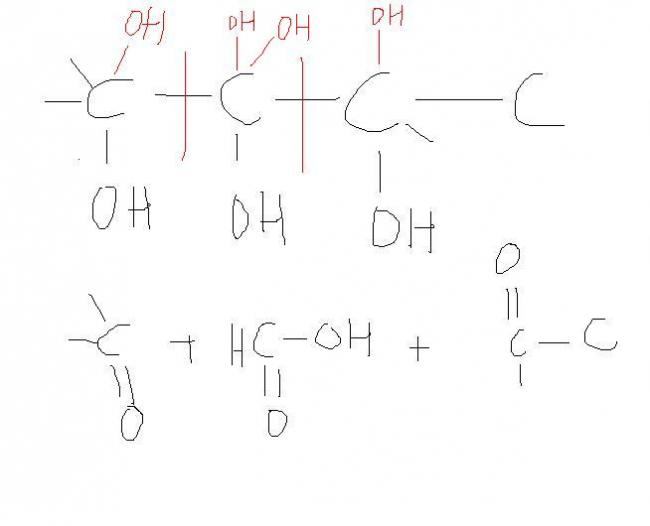
Oh you want general fn of NaIO4... i think eureka is right (maybe)..
well NaIO4 is an oxidising agent ...
jus like KMnO4....
o o
\\ //
I
// \\
oNa o
this is the structure.....
when there are two adjacent OH bonds naa... they attack with the H's
of OH ...
and something something happens (i dun remember, bcoz its not necessary)
and they oxidise.......