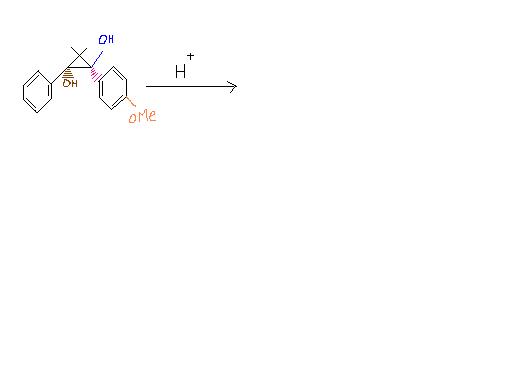
I think I know the answer ,but cant draw yhe diagram . can u plz tell me how to draw the diagram. Its not pinacol-pinacolone rearrangement.
10 Answers
Pinacol-pinacolone rearrangement? lekin 3 membered ring bhi hai..yeh question pehle kiya nahi hua?
But why it's not pinacol pinacolone rearrangement ? 1,2 - diol ????
pratik use MS Paint in Start->Accessories->MS Paint, make the image, go to File->Save As and save it as a JPG file. Then upload the image here.
I'm feeling kinda lazy to work this out, but it's not gonna be PP rearrangement, so too I think now. Proton will attack the three membered ring instead, carbocation will form.
Debo ko aane do..in his zeal he will immediately make a diagram and post for us :P
Here, H+ will attack the (blue coloured) OH bcoz e- rich group will migrate in preference. Am I correct Pritish ???
Asish ne sapnon ki duniya se jaga diya mujhe..Lol. aieeee has solved it beautifully.
The benzene ring undergoes normal resonance before H+ attacks. Since a + charge forms adjacent to a carbon atom of the cyclopropane ring, ring expansion takes place to accommodate the charge. Now the + charge occurs on the carbon atom holding the brown -OH. H+ can't attack brown -OH because + charge is now already present via resonance and then ring expansion. Uptil resonance the process is reversible but ring expansion is highly favoured and pushes the process forward.
Resonance occurs again and a bond forms between the + charged carbon and a negatively charged carbon on the benzene ring. Aromaticity is lost temporarily. Now the other -OH is attacked by H+ and it leaves. The remaining negative charge forms a pi bond as a bond simultaneously cleaves inside the cyclobutane ring to relieve the + charge caused by -OH leaving. Aromaticity is regained by the negative charge forming a pi bond. Then we have normal tautomerism after both rings are fused.
Weird question..I have no idea how he derived the answer but it seems kinda plausible to me.
Thanx Asish , aieee and Pritish [1]
And sorry Pritish for wasting your time.