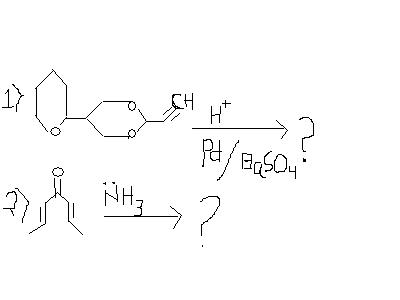
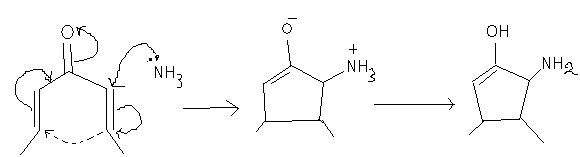
In 2 is it Na/NH3 or just NH3?
7 Answers
Manmay kumar Mohanty
·2010-02-27 03:52:17
I THINK IT IS NH3.. ITS LP WILL ATTACK CARBONYL GROUP.....
rishan chattaraj
·2010-02-27 23:53:32
in the first question the only change that(i think!) will happen is : the triple bond will get converted to the double bond as lindlar's catalyst has been used.
will anything other than this occur??
Pritish Chakraborty
·2010-02-27 23:54:58
No, in 1st question there is a proton too. Hydrolysis of ether groups into separate alcohols takes place, then reduction of alkyne.
Pritish Chakraborty
·2010-02-28 01:52:44
Manmay I don't know..it looks like an imine-formation reaction, but there is no acid present...neither is it Na/NH3(trans hydrogenation agent)