Is (d) the answer?
Solvolysis will follow an SN1 mechanism here, and we have to compare the stabilities of the carbocations formed on chlorine leaving in each substrate. The tertiary carbocations are stabilised by resonance in each molecule, and at the para position the + charge is stabilised by p-orbital delocalisation of the cyclopropane group.
It doesn't expand the cyclopropane ring as the aromatic character of benzene would be lost if it did.
The methyl groups at the meta position do not contribute by resonance but by the inductive effect, which is active at even the meta positions. Thus the intermediate of III would be highly stabilised.
III > II > I > IV
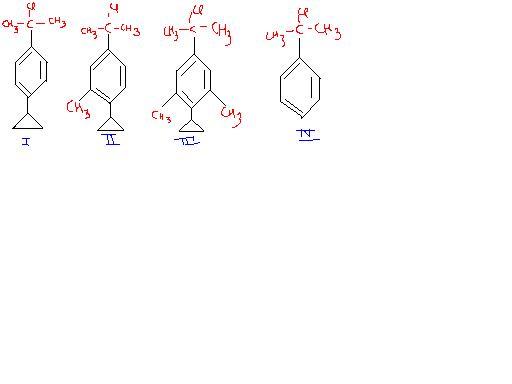
The decreasing order of reactivity of the compounds given below towards solvolysis under identical conditions is:
(a) II >III >IV>I (b) I>II>III>IV
(c) IV>III>II>I (d) III >II >I>IV
-
UP 0 DOWN 0 1 5

5 Answers
Pritish Chakraborty
·2009-10-27 05:04:34
Tush Watts
·2009-10-27 05:28:46
Yes right answer Pritish. Thanx a lot !!!!!!!!!!!! [1][1]
But one doubt, I just need to know whether for solvolysis, we need to considerSn1 or Sn2?????
msp
·2009-10-27 07:10:04
i think it depends on the alkyl halide tushie,if it is a secondary halide only it depends upon the product that we want.
Pritish Chakraborty
·2009-10-27 07:13:50
Depends on the halide used. If primary or methyl, it will prefer SN2.