106
106bhaskar see #7. the answer is (C)
 106
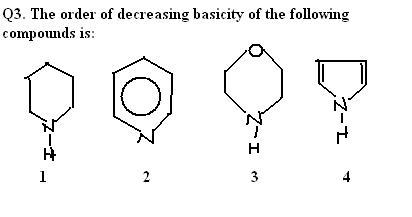
106ohh im sry dimensions is correct... for Q3. 1>3>2>4
 1
1the ans for the 3rd 1 acc.to me should be 3>1>2>4 bcoz more less the delocalisation moer basic is the compound & also more the no.. of lone pairs of elec.. more basic it is....
wats ur ans??? mr.asish
 1
1ashish
wat's ans. of 3rd one!!!!!
 106
106@manish sir: the pKa values of methanol = 15.5 and pKa of water = 15.74 i found on Wikipedia and obviously phenol is more acidic than methanol .. so it should be (c)
pKa value of water can be easily calculated to be 15.74
as Ka of water = [H+][OH-]/[H2O]
= (1*10-14)/(55.55)
So, pKa of water = 14 + log(1000/18) = 17 - log(18) = 15.74
pKa of methanol = 15.5 from wiki
http://en.wikipedia.org/wiki/Methanol
 1357
1357For first one I think it should be (a) only
Regarding pKa values, try to find it from a good source (I din't find any)
 106
106ya that must be p>o>m mite be a typo ...
@dimensions, john and bhaskar: out of all aliphatic alcohols, only methanol is stronger than water (acidic) all other aliphatic alcohols are weaker check post #7
 1
1basicity for nitrogen containning compounds follow order sp3>sp2(locallised)>sp2(delocallised)>sp
& for 1st as far as i remembere, water is moreacidic than aliphatic alcohols
 1
1for 1st a) must be correct as said by bhaskar & john
for 2nd mine too is comming p>o>m & for 3rd is it 1>3>2>4 ??
 1
1ans is A ....
bcoz CH3OH is highest basic then H2O & then phenol
do u agree????
 106
106For Q1. see this:
http://www.goiit.com/posts/list/organic-chemistry-my-question-is-why-ch3oh-is-more-acidic-than-41366.htm
and
http://wiki.answers.com/Q/Why_is_methanol_more_acidic_than_water
quoting wiki answers:
Methanol is only slightly more acidic than water. The measure of the strength of an acid is its pKa value.
The pKa of water is 15.7 and for methanol it is 15.5.
The difference is primarily due to the stability of deprotonated negatively charge ion, or anion. When you deprotonate water you are left with the hydroxide ion, or OH-. When you deprotonate methanol (CH3OH), you are left with CH3O-. The larger the anion, the more the negative charge can be spread out (reducing the charge density) and this stabilizes negatively charge species.
Because CH3O- can better stabilize the negative charge than OH-, the resulting anion is more stable. However, they have nearly identical acidities!
 106
106Q2. acidity order among nitrophenols is in the order?
 1
1h2o ka acidic nature kam hai phenol se ??
[12]
pata ni tha...