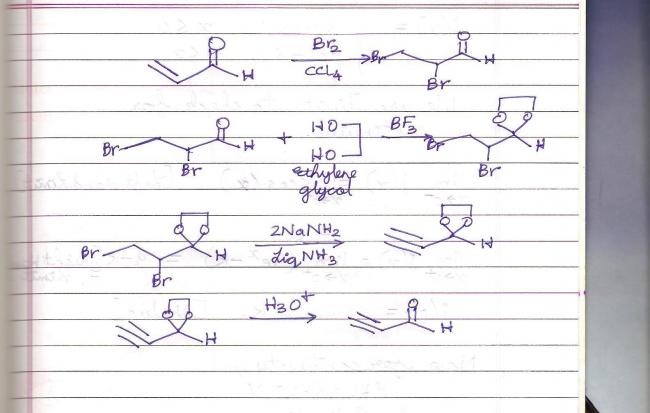
I guess, first reaction of acrolein with Br2 which will give
CH2Br - CH2Br - CHO , then reaction with NaNH2 will yield the desired product.
OUTLINE ALL STEPS INVLOVED IN SYNTHESIS OF
HC  C- CHO FROM ACROLEIN
C- CHO FROM ACROLEIN
-
UP 0 DOWN 0 1 7

7 Answers
@Ankur....NaNH2 in the presence of NH3 will not be gud..coz it can attack the carbonyl carbon
In that case, why shouldn't we protect the aldehyde group with dioxolane formation by glycol... the dioxolane formation ensures non-use of Bayer's reagent so alkene group in acrolein is unlikely to get adhered to this. After dioxolane formation, NH2- base ion will not act upon it (it becomes wary of it, and mercaptal formation get wary of acid).. in this way it can be definitely formed.
By the way, good thought govind , I completely ignored base action. Silly me.!
oopsy! Sorry Pritish, I took much time to type then your answer. your msg appeared just after refreshed page of submission.
again the same thing, the preceding post before me came quickly. :D .. I'm slow typist.. Silly me.