benzyl in both cases
which is more stable
1. allyl free radical 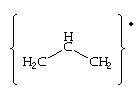 , benzyl free radical
, benzyl free radical
2.allyl carbocation 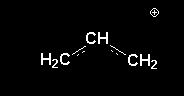 ,benzyl carbocation
,benzyl carbocation 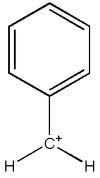
-
UP 0 DOWN 0 1 16

16 Answers
sorry yaar this is common mistake your answer if taken both as one question but one is corect & one wrong donot go on max. no. of resonating structure this will get fail in one case
VIshal dats wat i knw buddy.... rahul please explain clearly the answer...
i 'm giving one more day it is correct one think same tat u think the same i think when i met this question think on identical resonating structure ,hybrisation ,hindrance by which there is slight difference about 4kJ/mole
1. allyl free radical should b more stable dan benzyl free radical............as d aromaticity of benzene ring disturb by its free radical.......and it gt unstable.
2. benzyl carbocation should b more stable ,...coz iz hyperconjugation.
..............................may b it ri8. :(
benzyl is definitely more stable
there exists confusion only if t-butyl is involved.
where t-butyl ≈ benzyl.
no all of u r wrong u donot think of identical resonating structure of allyl in both case allyl win over benzyl as identical resonating structure has more contribution then no. of resonating structure in free radical the difference is very small as 4kJ/mole that can be neglected but it is wrong and in carbocation u donot consider steric hinderance which make C+ non planar & identical resonating structure also makes allyl better & great thing u got thatin many books it wrong given but in some foriegn writers have written comparable (almost same) but also written allyl is greater also u take help of minor effect as I effect and hyperconjugation
good question rahul and i am very much confident that u will score high in IIT and srinath it was a q u should'nt have done wrong
no of resonating structures is not the whole sole criteria u should think about the similarity of structures first
a common example phenol and benzoic acid
plz somebody tells me who decides to give color to the solutions ??
sorry buddy . but I'm damn sure on this.
for further clarification check this out.
http://www.cem.msu.edu/~reusch/VirtualText/addene1.htm#add1bc
gaurav.. I do all the pinks..
I will appreciate it if u can rather give the correct answer with your explanation too......
For this post, I still hold my pink on this post ...
(I am open to your explanation and discussion! ?...)