2. What is the Bond order of benzene?
Please Explain the Relative Stabilities of these compounds and arrange it in decreasing order.
Also, Please Explain the causes.
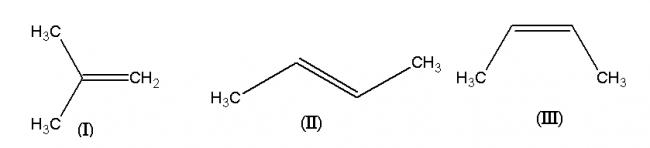
-
UP 0 DOWN 0 0 11

11 Answers
3. Please Explain me most Important factors with which acidity and basicity can be detemined/compared?
1) main cause of stability of alkenes is +H (hyperconjugative effect) and configuration (E/Z). Hence more substituted alkenes => more H effect hence more stability Further trans isomers are more stable than cis isomers due to steric factors.
Hence order is I > II > III
Note : I read somewhere that in case no. of substituents is same then if the substituents are at the same carbon, then its stability is higher. (hence I > II) . Pritish can give the reason
can give the reason
I need that!
Also Which form cis/transis stable of these compunds:
ICHCHBr and ICHCHI and FCHCHF
Found the bond order of benzene to be 1.5, But how does it come.
there are many ways to find it... a simole one that gives the value of BO is total no of bonds btwn two atomstotal no of resonating structures
dude
to check the stabilties of simple
see the no alpha H present
more the no of alpha h
more the stable is the alkene
simple funda
2>3 , wich u might be knowing ,
and for 1>2 i gav reason in #5 , isnt that sufficient ?
Yes. thanks.
@ Kalyan. They have same no/ of HC structures I think.