1) A
2) D
3) Compare n c which compounds r similar
2 changes at the chiral centre lead to retention of config
4) b ??? (Doubtful)
Ques 1) Among the following, the optically inactive compound is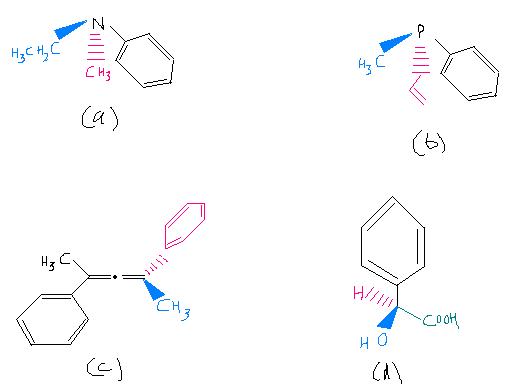
Ques 2) The binapthol (Bnp) is
(a) an optically active compound having chiral centre
(b)an optically inactive compound
(c) a meso compound (
d)an optically active compound without having vhiral centre
Ques 3) Which pairs of salts would have identical solubilities in methanol ?
Ques 4) Which of the following equilibria has the lowest dG° ??
1. (a) ??
2. (d) (the phenyl grps become non-planar due to steric factor)
1) A
2) D
3) Compare n c which compounds r similar
2 changes at the chiral centre lead to retention of config
4) b ??? (Doubtful)
utaara how (a), please point out the chiral carbon.????[7]
Something to do with amine inversions in 1?
In the solubilities question, I guess we have to check by R-S nomenclature, which ones are similar.
@Manmay and Pranav
The qs asks for optically inactive compound
In d there is 1 chiral C
So it is optically active
@ Asish u r correct
but in ques 2, there is no chiral centre , so how it is optically active ???
Anyone for ques 4 ???
compund 2 give atropisomerism
(optically active due to absence of plane and centre of symmetry)
Atropisomers are stereoisomers resulting from hindered rotation about single bonds where the steric strain barrier to rotation is high enough to allow for the isolation of the conformers.[1] The word atropisomer is derived from the Greek a which means not and tropos which translates as turn. The name was coined by Kuhn in 1933,[2] but atropisomerism was first detected in 6,6’-dinitro-2,2’-diphenic acid by Cristie in 1922.
Oki defined atropisomers as conformers that interconvert with a half-life of more than 1000 seconds at a given temperature.[3] Atropisomers are an important class of compounds because they display axial chirality. They differ from other chiral compounds in that they can be equilibrated thermally whereas in the other forms of chirality isomerization is usually only possible chemically.
Tushar zaroori thodi hai ki chirality chaiye for optical activity...we need one basic prerequisite, that is the substrate should be non superimposable on its mirror image. I suppose this happens here because the phenyl groups become non-planar as asish said. Molecules can be chiral without possessing a chirality center.
Wait..I found a little something that will help in this case.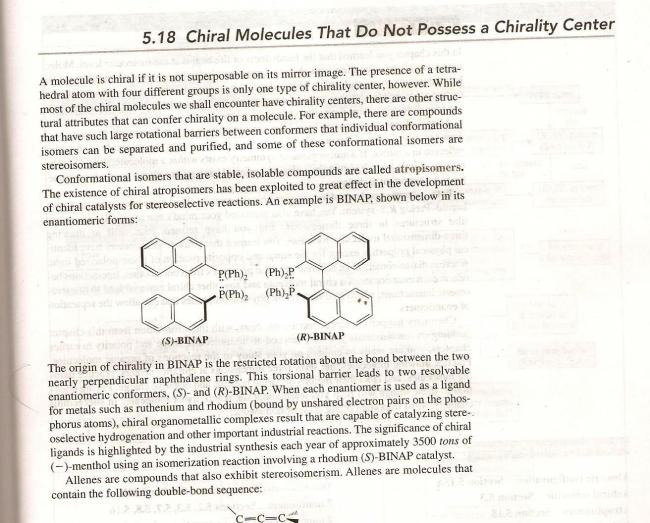
For question 4), in whichever six membered ring flipping leads to the more stable conformation, dG will be least..... I'm not sure of the answer as I haven't done these questions as much...