keep in mind the phenomenon of pyramidal inversion in trialkyl amines that makes them optically inactive....that happens in the third compound...but the protonated species of these amines are optically active .....thats all for this question !

Ques1) In the above figure, Compound A has two stereoisomers, but compounds B and C exist as single compounds.
Explain.
Ques2) 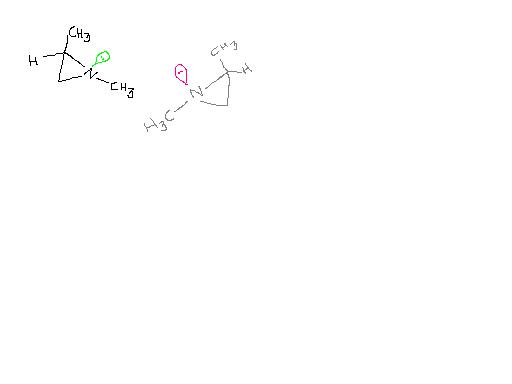
The enantiomers of 1,2-dimethylaziridine can be separated even though one of the “groups†attached to nitrogen is a lone pair.
Explain.
-
UP 0 DOWN 0 0 4

4 Answers
Asish Mahapatra
·2009-11-21 08:05:27
Q1. All the four groups are different so they exist as enantiomeric pairs. (A)
B --> there are two groups which are identical so carbon itself is achiral
C--> carbon is chiral but due to fast inversion process the two enantiomers are non-resolvable
Q2. possibly has something to do with restricted inversion ... thinking
Debotosh..
·2009-11-21 08:25:44