yes
question is in the image. please also explain.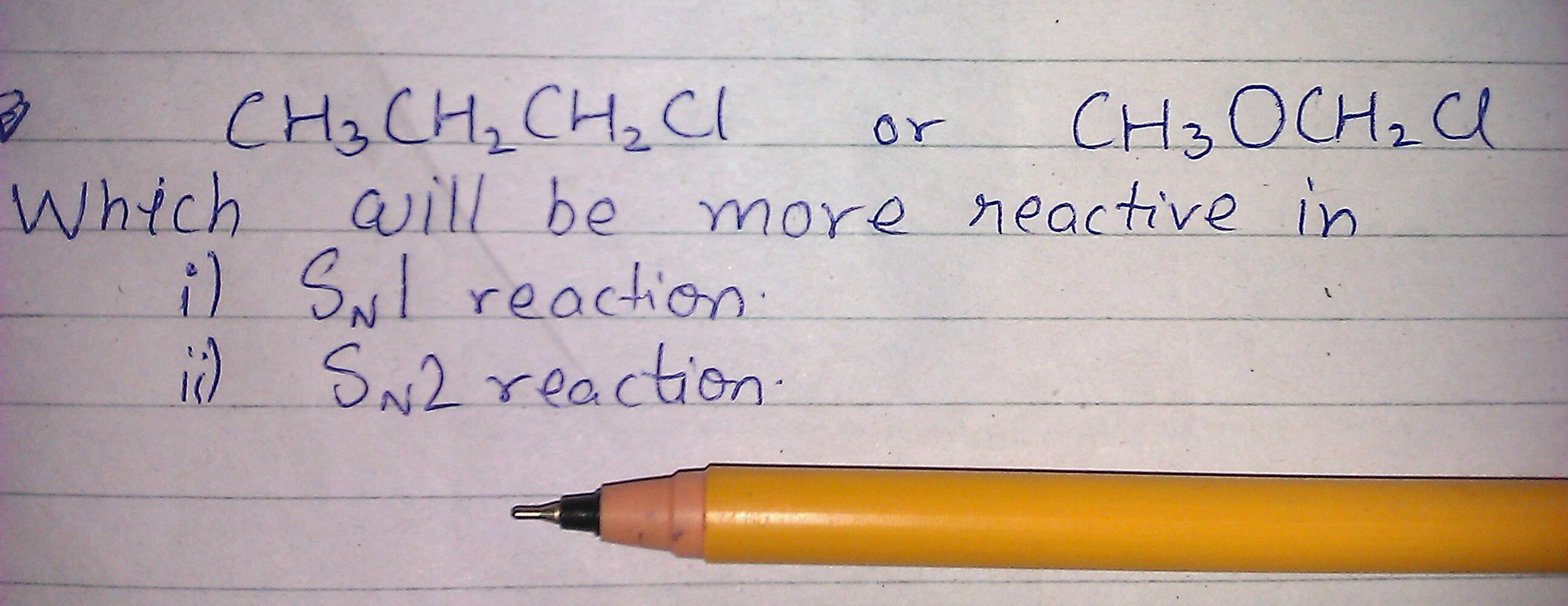 question is in the image. please also explain.
question is in the image. please also explain.
-
UP 0 DOWN 0 0 2

2 Answers
Swarna Kamal Dhyawala
·2013-01-26 04:32:46
Sn1 reaction → CH3oCH2 Cl
Sn2 reaction →CH3CH2CH2Cl
 Anurag Ghosh Why Swarna Kamal??......I mean how did u say dat??Upvote·0· Reply ·2013-02-04 05:16:38
Anurag Ghosh Why Swarna Kamal??......I mean how did u say dat??Upvote·0· Reply ·2013-02-04 05:16:38 Swarna Kamal Dhyawala @anurag propyl group has +I effect so it will easily lose chlorine and form 1 degree carbocation and also after 1,2 methyl shift it would form 2 degree stable carbocation . so the reaction would be carried out in 2 steps therefore it wud be sn1 reaction but in the second case it is not easy to form carbocation (due to presence of oxygen )so it wud follow sn1 mechanism
Swarna Kamal Dhyawala @anurag propyl group has +I effect so it will easily lose chlorine and form 1 degree carbocation and also after 1,2 methyl shift it would form 2 degree stable carbocation . so the reaction would be carried out in 2 steps therefore it wud be sn1 reaction but in the second case it is not easy to form carbocation (due to presence of oxygen )so it wud follow sn1 mechanism Anurag Ghosh In that propyl chloride,d rearrangement of carbocation has to be accepted as a step??......I mean as soon as d carbocation(stable) is formed,it should immediately get joined to a nucleophile na??.....Sn2 reaction is a 1 step reaction and 2nd order reaction na?
Anurag Ghosh In that propyl chloride,d rearrangement of carbocation has to be accepted as a step??......I mean as soon as d carbocation(stable) is formed,it should immediately get joined to a nucleophile na??.....Sn2 reaction is a 1 step reaction and 2nd order reaction na?
 Swarna Kamal Dhyawala ya i m sorry by mistake i wrote wrong
Sn1 reaction -CH3CH2CH2Cl
Sn2 reaction -CH3oCH2Cl
Swarna Kamal Dhyawala ya i m sorry by mistake i wrote wrong
Sn1 reaction -CH3CH2CH2Cl
Sn2 reaction -CH3oCH2Cl 