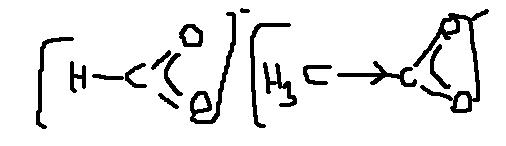False?
9 Answers
Shreyan
·2009-06-10 11:33:10
yeah, shud be false...
more acid. strength of formic acid is due to lesser +I (bec there are no alkyl groups)
JOHNCENA IS BACK
·2009-06-11 00:04:38
and ans. is true.....it is 100% correct..........can u guess why!!!!!!!!
if not tell i will reveal..............the fact!!!!!
JOHNCENA IS BACK
·2009-06-11 06:31:33
i feel that here one point should be noted............
+I is responsible for lesser stability of acetate ion......right!!!!!!!
but it does not have any role for formate ion........for formate ion we have just one thing resonance stabilization........so.............
wat do we conclude :
+I in acetate ion makes it lesser stable
or putting it differently,
as the statement given in ques.