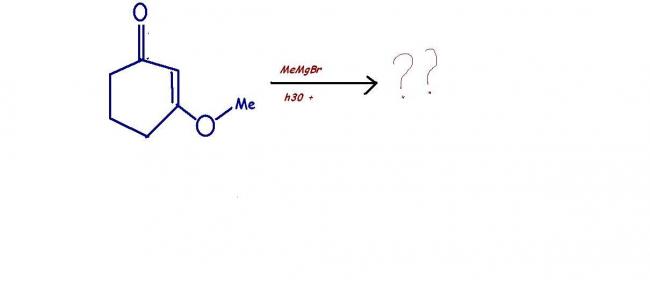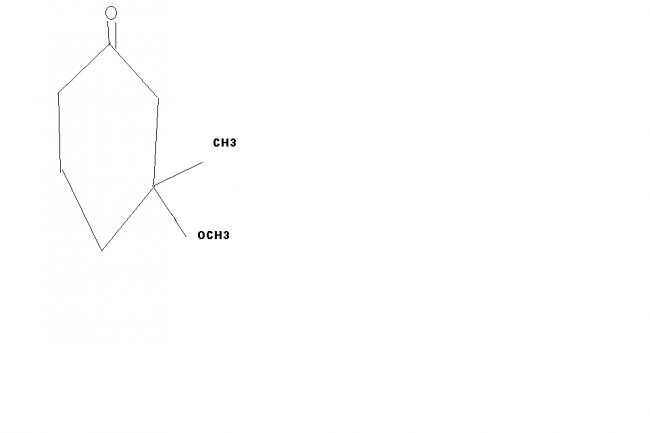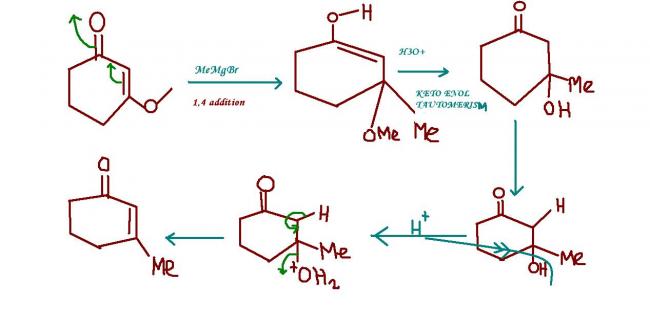
34 Answers
i am too tired today.....i have an even busier schedule tomorrow....the mech is a bit complex and needs explanation...i will try tomorrow or day after tomorrow!
@ organic
are u sure the ans given by u is correct bcoz i am not getting the same ans
yes man.......i am sure!!!!!!!!! this is a complicated mech and u might have made a mistake somewhere!!!!!
is that i am not worthy of the getting enlighten by u or .............
but after hydrolysis in acidic medium no heat is involve som how can one say whether dehydration occurs or not as nothing is specified regarding conc of the acid
and in 1,4 addition as u are doing it will follow SN2' so in case of three degree will there be any difference or not ???
so..........
@gagar.......do you know that tertiary alcohols can undergo dehydration under very simple conditions.....here only the acidic medium is enough !!!!
grignard reagent has a strong nucleophilic part(the alkyl part----actually a potential nuclephile) which goes for 1,4 addition to achieve greater stability of ts and final products !!
but the reaction follows SN2' ( in case of 1,4 ) so0 in case of three degree is it good to make the attack at more hindered C atom
answer by organic is right . enjoy the discussions :)
@ gagar bhaiyya is it SN2 ???? I mean it's a alkene there . hopefully this is ok or maybe am I making a mistake somewhere ?????[7] lost touch of this thing got to revise
An alcohol is a always a product when Grignard's reagent reacts with C=O group.
Will Me-OH and cyclo hexanol be the products ???
i feel this is grignard reagents rxn. so the oxygen will be replaced by -OH TO MAKE THIS KETONE TO A 2O ALCOHOL .. pls tell me is my answer correct
no my dear ankur..........the reaction is not such an easy one.....it requires a bit more thinking on the concepts of organic.....your answer to my question on b.v.oxidation reveals that you are just too eager to comment...but not with much sense!!!!!!!
I WILL GIVE YOU A HINT: you can use only 1 mole MeMgBr but there is excess of H30 + !!!!!!!!!
organic i am just giving a try
if we use 1 mole of memgbr it is just grignard reaction,but we have excess of hydronium ions which have gr8er possibility of interacting with the oxygen atom in -O-Me gp so the the hydrogen atom replaces the methyl gp and the Me-0H will also get formed see this reaction yields a highly stable product as u can see the bond energy of the final rxn product is very high, and also methanol is stabilised by the solvation effect.If u have used a catalyst then there is a chance for the reduction of C-C double bond.
i am a little bit confused if michael attack is possible with grignard reagent .....
surabhi......absolutely nowhere near the right one!!!! better retry!!!!!!
i cannot understand what you are saying@msp...... answer in a straightforward manner....no jokes needed!!!!!!
i havenot got the correct answer till now!!!! so more answers are welcome !!!