@Debo
Isn't it understood..? Like someone so graciously said to me when the hydrogens weren't mentioned in a question.
What shud be the major products "A" & "B" in the foll. simple cases....?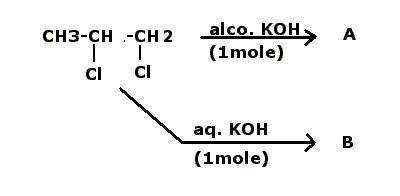
(P.S. I know, too simple, ...yet, awaiting replies to clear the confusion once n fr all [1])
-
UP 0 DOWN 0 1 22

22 Answers
Oho..I missed out Saytzeff Rule. CH3 will provide hyperconjugation. Is there anything else to be mentioned?
Okay, so tht way, it'll get 3 H's fr Hyperconj. as compared to only 2 in the latter case, Hence 1-Chloropropene ??
double bond preferentially forms due to elimination of hydrogen atoms from the carbon atom containing lesser hydrogen atoms....in other words...you try to develop the more substituted double bond......thats saytzeff rule for you ! [68]
But am still confused ki 20 Carbocation banane ke baad, why = bond can't be formed with its left side carbon ?
Arrey sorry yaar Debo, fr those Stupid xtraa Hydrogens (Edited now !)...(brain was so messed up with the Qn thts why [3])
oh yeah got it.......
i guess its the simple questions dat confuse us the most
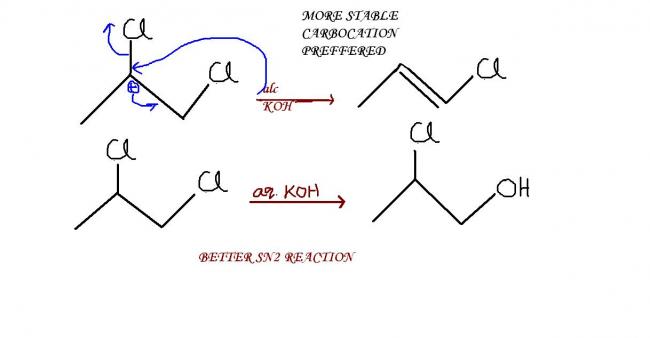
alcoholic KOH is a reagent for elimination, here dehydrohalogenation..proceeds via more stable carbocation path! hence no SN2 !
aqueous KOH is a reagent for substitution....goes via SN2 !
A- 1-chloro propene
B-2 chloro 1 propanol
.....(assuming that no carbon atom can exist with valency 5) [4]
@Shreyan-For SN1 rxn the reagent should be neutral. Aq. KOH is not neutral. So SN1 rxn will not occur.
without referring to any of the comments above, what i can see in the question is that it is wrong....give the correct structure first of all !
[3]...ok ok...really silly mistake of mine (actually, mum was calling for dinner....so..... :))
and @pritish...nice explanation..:)
Alcoholic KOH is strong base. E1 and E2 have same reactivity order, because tertiary carbons have max hyperconjugation structures. Thus 2-chloropropene is favoured.
Also aq KOH is an unhindered base, it will prefer direct mechanism. Which occurs in SN2.
You know what? I could be stupid, but alc KOH in first question can abstract a proton from CH3, forming carbanion which attacks the 3rd carbon and Cl leaves, forming three membered ring. Kinetics favours it. So that's possible too.
Waise, i don't know ny right or wrong answers fr these....'tis smthing which clicked while i was solving a Qn...
Jaldi-baazi mein keh diya hoga shreyan ne [4]...
par arshad, why did u replace the beech waala Cl..???
@shreyan look closely if sn1 takes place in b wat u are saying will be formed or not????
remember a carbocation is formed in sn1....
How are v deciding b/w 2-Chloropropene & 3-Chloropropene...??
n fr 2) SN2 occurs toh , 10 waala Cl shud be replaced more easily ??