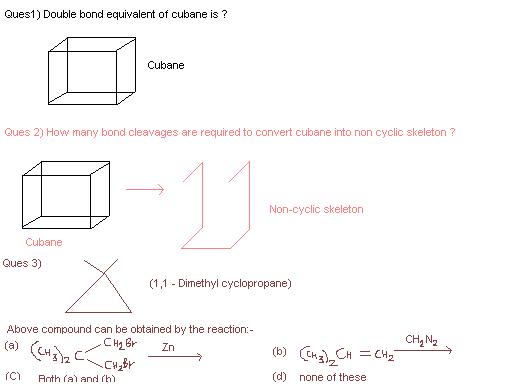
1. C8H8 so DBE = (18-8)/2 = 5
2. 4
3. C
9 Answers
Haan asish I also made the same silly mistake before you lol...then I noticed one cyclobutane ring was still there :P
Pity this thread died....I guess I'll give my take now.
The first two questions have been solved correctly so I'm not doing those.
Q3 is very simple. The correct answer is (c). In the first option, zinc(in ethanoic acid solvent) abstracts syn-vicinal halides to form a C-C bond. This could be a single bond if no bond is present, a double bond if a single bond is present, and a triple if double is present. So cyclopropanation does occur.
In the second option, diazomethane degenerates to form a carbene which cyclopropanates the double bond.
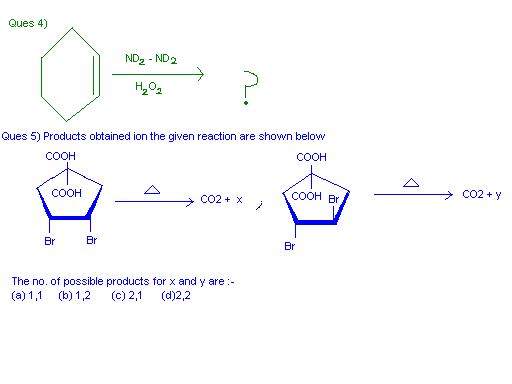
Q4 is however not as simple as it seems. It is not a hydroboration-type reaction. Note the difference : in hydroboration, boron has a vacant orbital which can be used. Nitrogen has no such vacant orbitals here.
Infact, hydrazine is used as a reducing agent. It is first oxidized to a diimide by an oxidizing agent(that being H2O2 here) and this diimide hydrogenates the double bond in a syn manner. Just that here it would be a deuteration instead of a hydrogenation.
This is also known as a transfer hydrogenation.
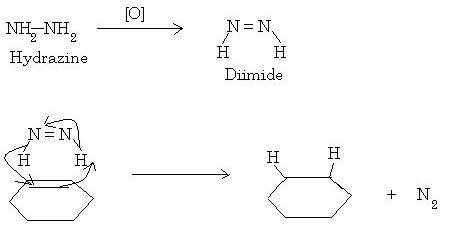
Just replace H with D.
Q5 is simple too. Heating causes the obvious decarboxylation of the beta-keto acids in both cases. Thus only one -COOH group remains. After that a lactone forms due to heating of gamma-halo acids. Due to syn configuration of the bromine atoms in the first substrate, the product formed is same whichever bromine -COO- is able to knock out. But due to anti configuration in the second substrate, the product will not be the same.
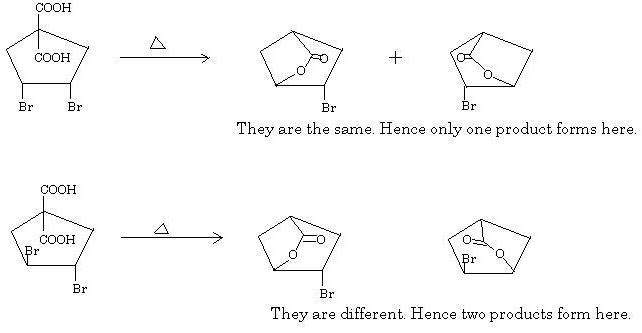
Hence the answer is (b) 1,2.