no...otherwise i would not have cared to put it up here !
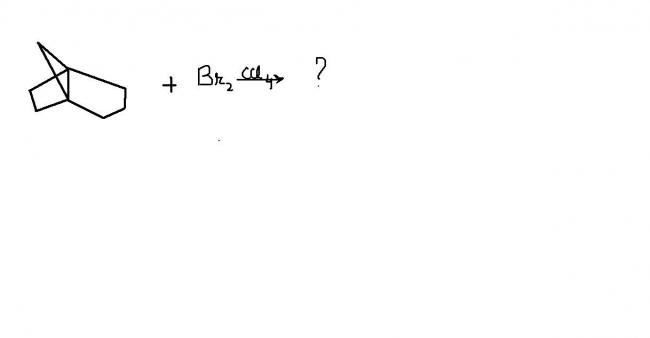
...really good question,,,,took me some time to get into it !
-
UP 0 DOWN 0 1 12

12 Answers
There will no reaction.
This is a rule ( I think Bredt's rule), that in bridge compounds, because of too much strain, double or triple bond is not formed.
Therefore no reaction.
where do you spot the formation of double or triple bond ??? ...strange !
Double or triple bond toh elimination hua bhai...that is different. There is no leaving group attached to the bridge head carbon. Actually the concept you are talking about is that the bridge head carbon cannot be sp2 hybridised or planar. Due to ring strain, it is always non planar. SN aur E ke transition states aur intermediates mein sp2 hybridisation aata hai, isliye those reactions do not work out.
I am still wondering what to do here...debo can you give us a hint?
the hint makes it absolutely simple:
think of cyclic compounds and homologous alkenes !
maybe the Br2 will attack the highly strainde cyclopropane ring??