ya i knw......it is a reaction,.....but no idea if in syllabus or nt??
acetone can be converted into pinacole by????
→pinacole is not a name of specific compound na???
-
UP 0 DOWN 0 0 14

14 Answers
as far as i know pinacole is a reaction.....which is not in syllabus...[1][1]
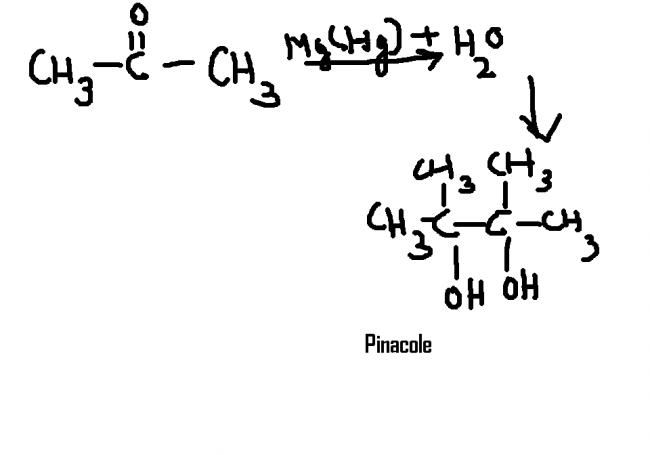
pinacole is formed wen acetone reacts wid Mg(Hg) + water gives pinacole. i m not very sure if it's in syllabus. bt if u want d mechn then i'l give u. also this is covered in aldehyde,keto ,and acids chap.
pinacole is in syllabus..(if its not then u shld study it as it may come in the form of a comprehension next year)
IT IS NOT IN SYLLABUS ........SO DONT STUDY IT...
and btw if u start studying every reaction in organic chem fearing that it would come in comprehension form next yr...then u wont be able to complete ur syllabus......organic chem is a very vast field,,,,and u can never ever complete it fully.....so just stick to the syllabus....leave the rest to ur luck[1][1]
sorry yes if u r upset wit hmy comments[1][1]
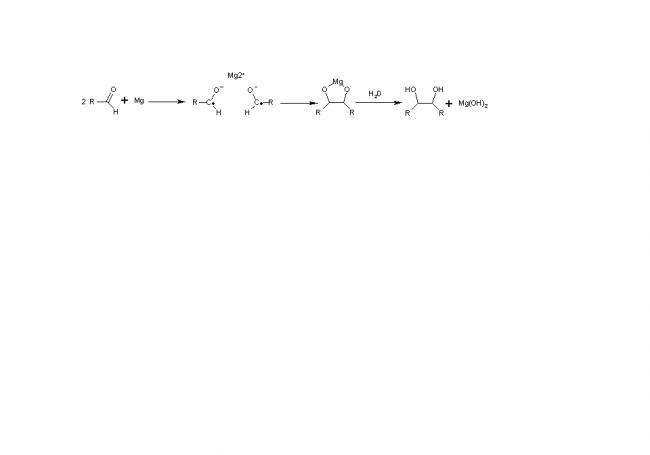
Reaction mechanism
The first step in the reaction mechanism is one-electron reduction of the carbonyl group by a reducing agent such as magnesium to a ketyl radical anion species. Two ketyl groups react in a coupling reaction yielding a vicinal diol with both hydroxyl groups deprotonated. Addition of water or another proton donor gives the diol. With magnesium as an electron donor, the initial reaction product is a 5-membered cyclic compound with the two oxygen atoms coordinated to the oxidized Mg2+ ion. This complex is also broken up by addition of water with formation of magnesium hydroxide. The pinacol coupling can be followed up by a pinacol rearrangement. A related reaction is the McMurry reaction, which uses titanium trichloride or titanium tetrachloride in conjunction with a reducing agent for the formation of the metal-diol complex, and which takes place with an additional deoxygenation reaction step in order to provide an alkene product.
i am talking of pinacole pinacolone rearrangement reaction..but the reactions u r posting doesnt seem like it :O..
pinnacole is simply a reaction n basically a mechanism nthng much its nt a compund ... its applied on compounds
in dis reacton we doesnt use.. charge /radius ≠sigma..
dats it d pinnacole formation
mag.!!!!
i didnt understand u????wht ..'charge/radius..."???
and pinnacole...is a common name for a compound wch has to -OH grps...
pinacole means highly substitued alcohol .........
n we hav d rxtion
pinacole ----- converted to pinacolone in syllb
pinacolone is highly subtitued ketone
Pinacol itself is produced by magnesium reduction of acetone, probably by way of a ketyl intermediate. Since the diol is symmetrical, protonation and loss of water takes place with equal probability at either hydroxyl group. The resulting 3º-carbocation is relatively stable, and has been shown to return to pinacol by reaction in the presence of isotopically labeled water. A 1,2-methyl shift generates an even more stable carbocation in which the charge is delocalized by heteroatom resonance. Indeed, this new cation is simply the conjugate acid of the ketone pinacolone, which is the product of repeated rearrangements catalyzed by proton transfer. Each step in this rearrangement is potentially reversible, as demonstrated by the acid catalyzed dehydration of pinacolone (and pinacol) to 2,3-dimethyl-1,3-butadiene under vigorous conditions.
mechanism: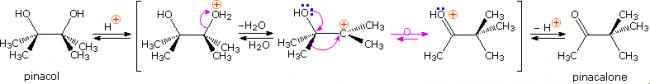
some selected pinacole rearangement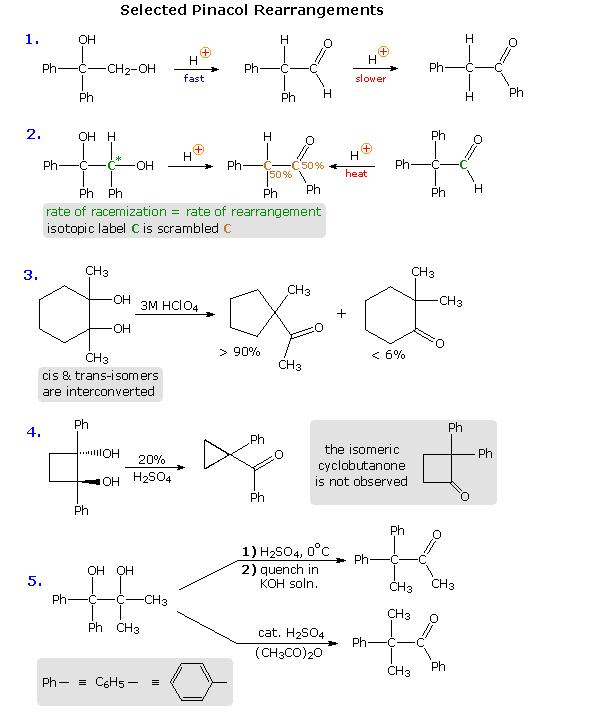
you know, you guys can contribute to wikipedia here
http://en.wikipedia.org/wiki/Pinacol