more reactive than benzene ! ------ NOT ALWAYS
18 Answers
inorganik benzene is more reactive than organik one !
r they mesomers ?
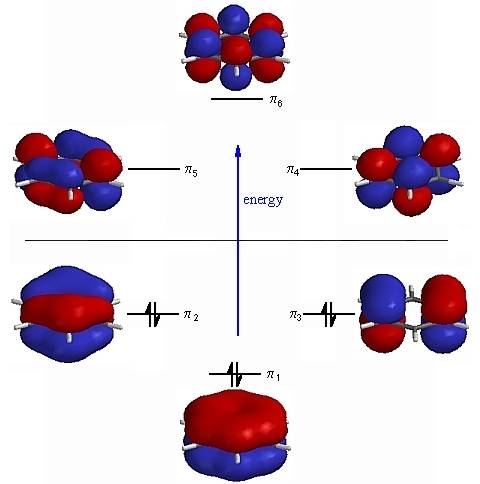
FOR ELECTROPHILIC SUBSTITUTION REACTION,
BENZENE IS MORE REACTIVE THAN BORAZINE.
check my post again!wat do u see it is resonance structure na!thendefinitely it shows resonance!
arrrrrrrre dude ,
its u who r in hyyypertension
i dont want history of it
just
wheteher its shows resonance like benzene or not?
listen dude, wat we are doing is last minute cramm-up..its only gonna make us panicky..jus feel confident of urself...
anyways,
as said ioragnic benzene or borazole, is N3B3H6...
its iso-electronic with benzene, more reactive than benzene,
prepared by reaxn of B2H6 with NH3..conditions mat poocho...
ALL THE BEST
i know
benzene is
is inorganik benzene to mesomerik , do it too show resonance ?
betn N n B ?
Coz its structure is like benzene....with alternate co ord bonds between B and N.
d structure is in d shape of BENZENE and its inorganic ,...
hence d name...inorganic benzene