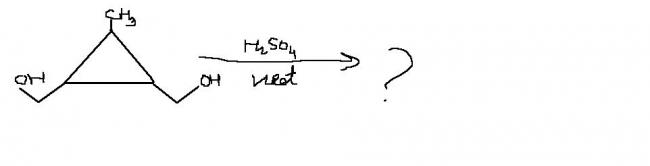
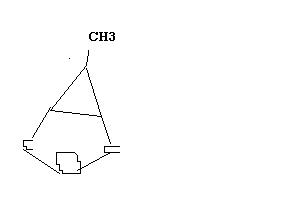
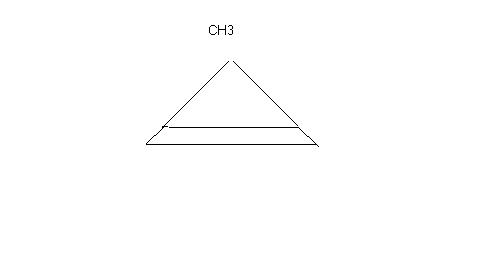
no....6 membered ring..............
14 Answers
OH SHIT!!!
THAT MEANS EVEN THE CH3 AT THE TOP GETS ENGULFED!!!!
is it benzene???
OH K....
now i better stop guessin.... LOL......
Let the chem scholars try it then,
i m not bad at it.... thats it.... LOL......
nah ORGANIC is tooooooooo vast ne so it bcums real tuff
noo..... sankara and ranbir........................
@ sankara I too got the same product as yours ....but answer is different
nice Q buddy . I still am not able to figure out how u get 6 membered ring me getting only 5 membered ring. unless it's some rearrangement. other than MagnerWeermein. so , give product da. atleast we should try the mechanism. thanks dude.
two such molecules will rearrange to give something something.....may b a six membered ring(as already said by eureka..) ...only this much i can tell...
i think dat the rxn would end in the expansion of the ring and so dat it can be free from Bayers strain and also my product suffers from Bayers strain , i know dis but i dont know to guess the product eureka