I think 1st 1 will b less as compared to 2nd one as 1st 1 displaces primary hydrogen while 2nd one dislpaces 3 degree hydrogen..
Am i correct??
Chlorination of isobutane gives mixture of 2 isomeric monochlorides
CH3
|
CH3-----CH-----CH2-----Cl
CH3
|
CH3-----C----CH3
|
Cl
I think 1st 1 will b less as compared to 2nd one as 1st 1 displaces primary hydrogen while 2nd one dislpaces 3 degree hydrogen..
Am i correct??
no dude...the first one is formed in major proportions ...look at the reactivity table of chlorine and bromine...that gives the answer !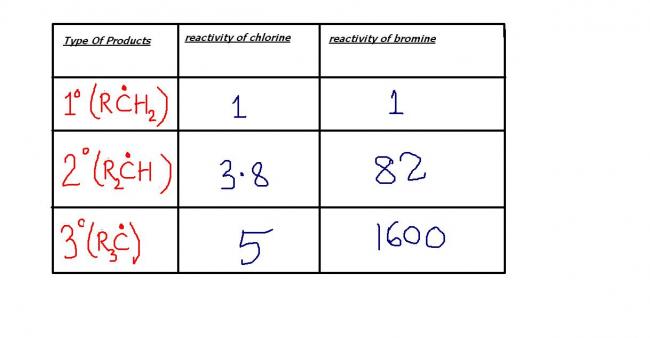
due to small difference in reactivity between 2° and 3° radicals,chlorine prefers to attack 2°radical and avoid structural hindrance ! tertiary product would have dominated for bromine---the table gives us that perfect data !
simple man chlorin is more reactive n less selective so it will attack region where there is more probability n bromine is more selectie than reactive so it will attack a region where stability is more..